45 electrolysis of water lab answers
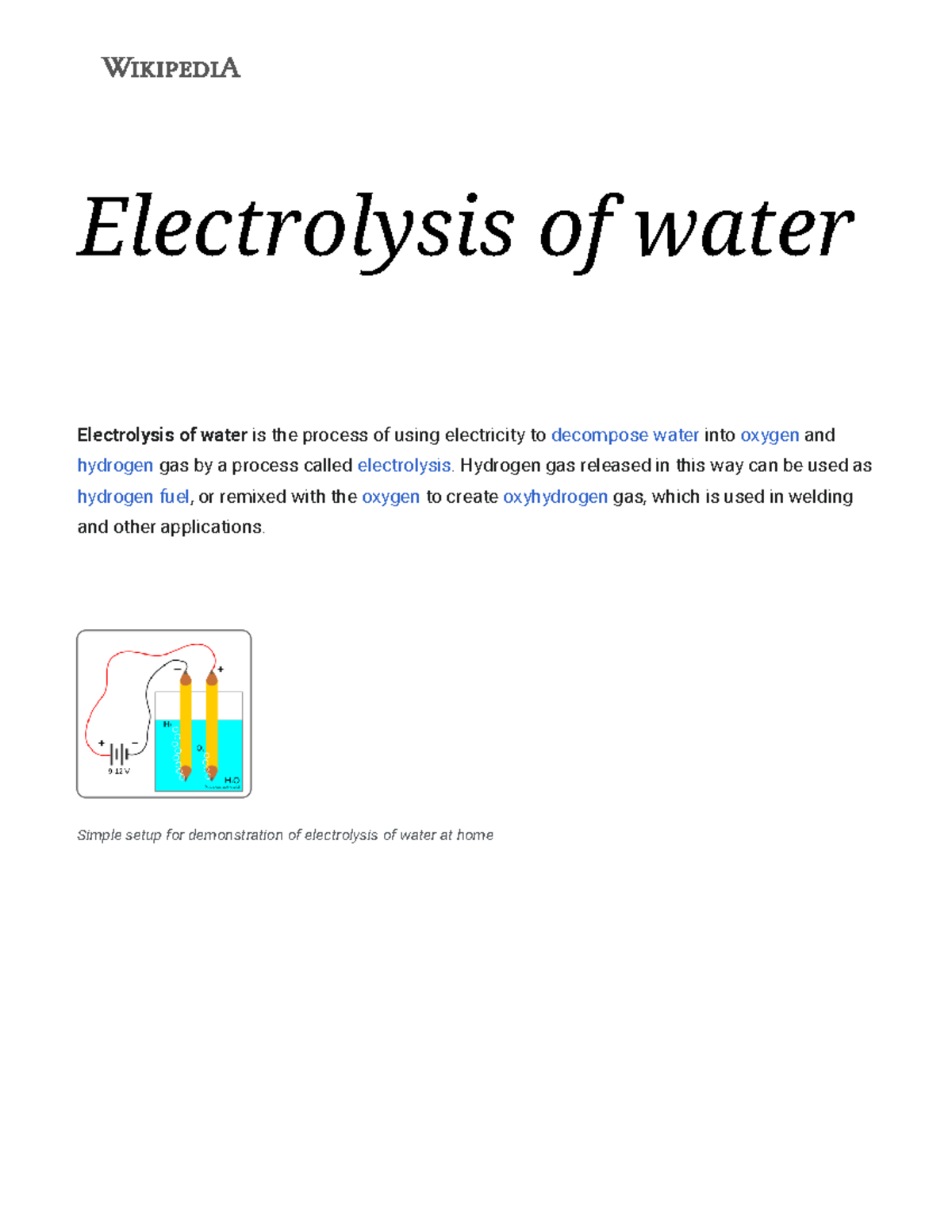
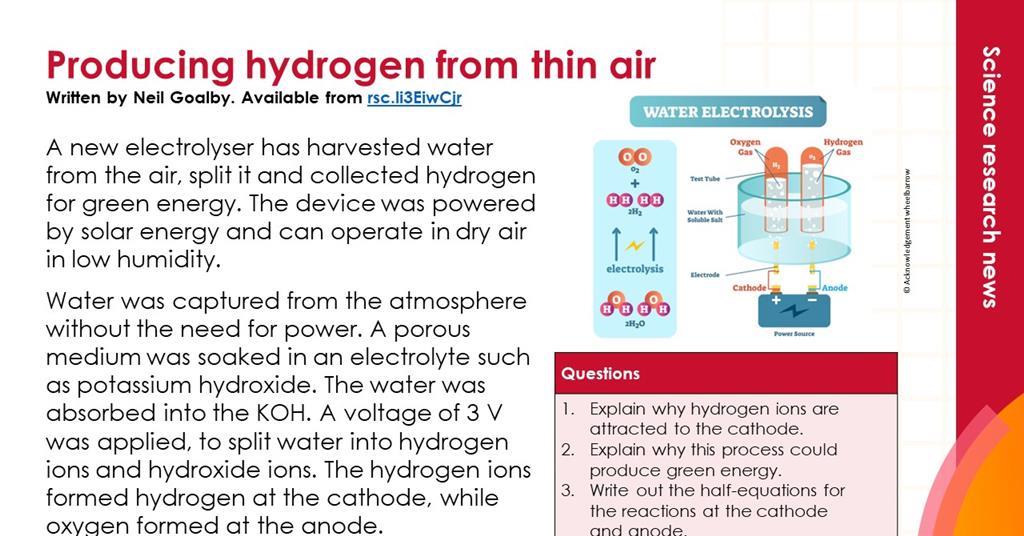
Electrolysis of Water Lab Flashcards | Quizlet Electrolyte a solution that conducts electricity solution liquid mixture of 2 or more substances mixed together Electrodes metal rods immersed in the solution Cathode negative electrode (black wire) Anode positive electrode (red wire) Battery power source contains energy stored in the form of chemical energy Ratio of hydrogen gas to oxygen gas 2:1 Electrolysis of Water Experiment - Splitting Water | HST Electrolysis is the process by which an electric current is passed through a substance to affect a chemical change. The chemical change occurs when the substance loses electrons (oxidation) or gains them (reduction). In the two experiments listed below, the first reactive substance is water and the second one is a copper sulfate solution.
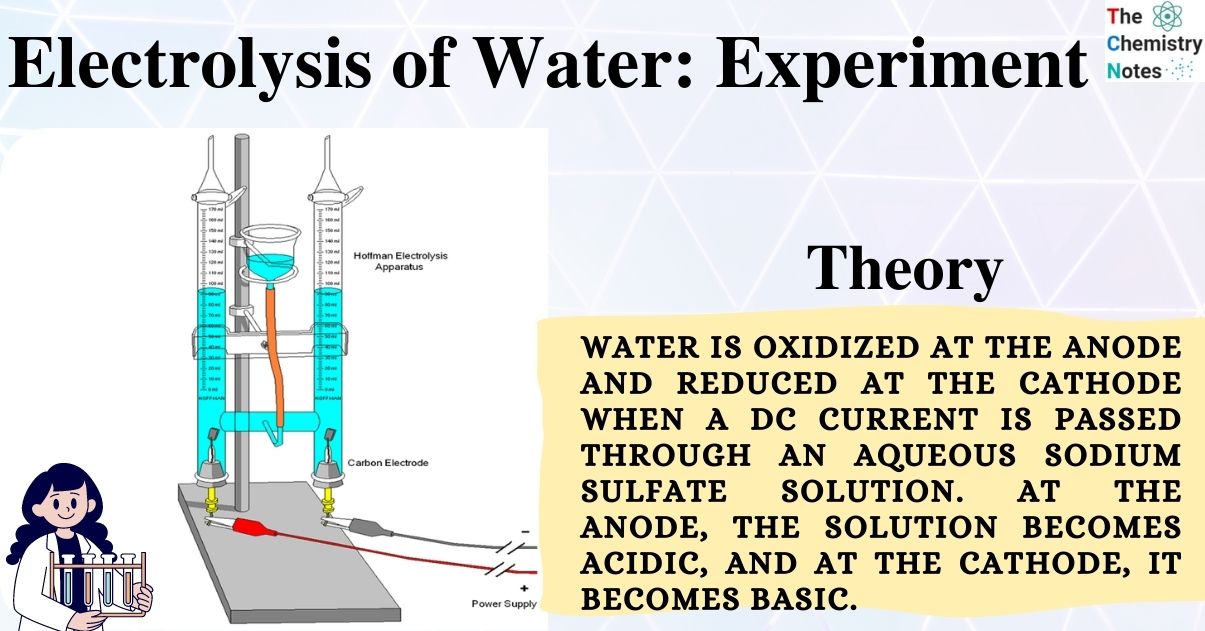
Electrolysis: Splitting Water - Stanford University Electrolysis: Splitting Water Teacher Version In this lab you will use a battery to perform electrolysis, or chemical decomposition, of different aqueous solutions (like water) to produce gases (like hydrogen and oxygen in the case of water). You will measure the volumes of gas produced and compare this to the predicted ratios from chemical ...
Electrolysis of water lab answers
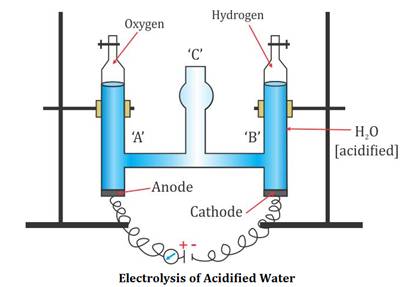
Activity: Electrolysis of Water | manoa.hawaii.edu ... What is the chemical formula of water? Answer the following questions based on the gas formed at each electrode. a. How much gas formed at each electrode? b. How do the volumes of the gases compare? c. How might you explain any differences? d. How does this provide evidence for or against the chemical formula for water? Electrochemistry: Electrolysis of Water Lab Electrolysis Solution X Post-Lab Questions: 1. Suggest an explanation for the initial indicator color of the electrolysis solution. 2. Describe at least three observations that indicate a chemical reaction has occurred during the electrolysis of water. 3. What are the two functions of the pencil-lead-electrodes? 4.
Electrolysis of water lab answers. Electrochemistry: Electrolysis of Water Lab Electrolysis Solution X Post-Lab Questions: 1. Suggest an explanation for the initial indicator color of the electrolysis solution. 2. Describe at least three observations that indicate a chemical reaction has occurred during the electrolysis of water. 3. What are the two functions of the pencil-lead-electrodes? 4. Activity: Electrolysis of Water | manoa.hawaii.edu ... What is the chemical formula of water? Answer the following questions based on the gas formed at each electrode. a. How much gas formed at each electrode? b. How do the volumes of the gases compare? c. How might you explain any differences? d. How does this provide evidence for or against the chemical formula for water?










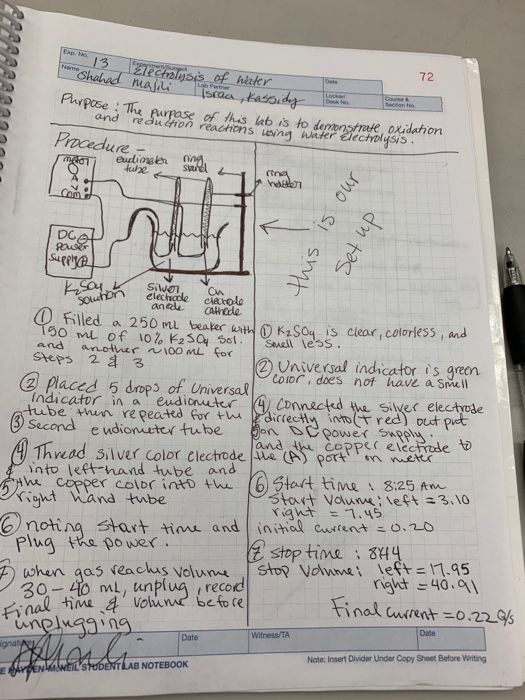


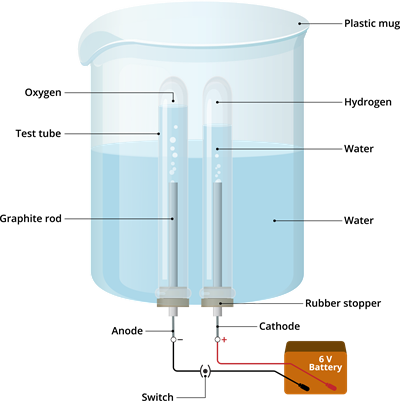




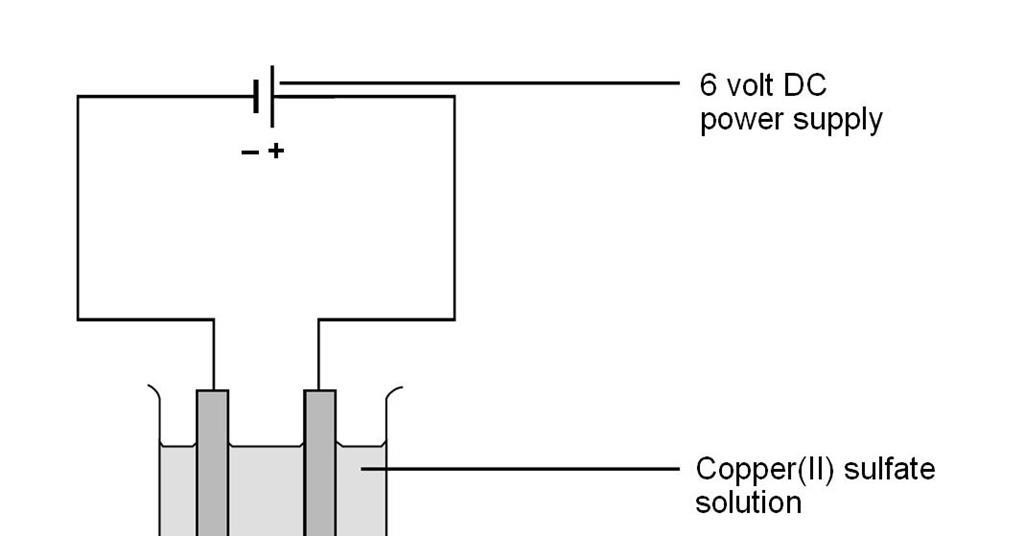



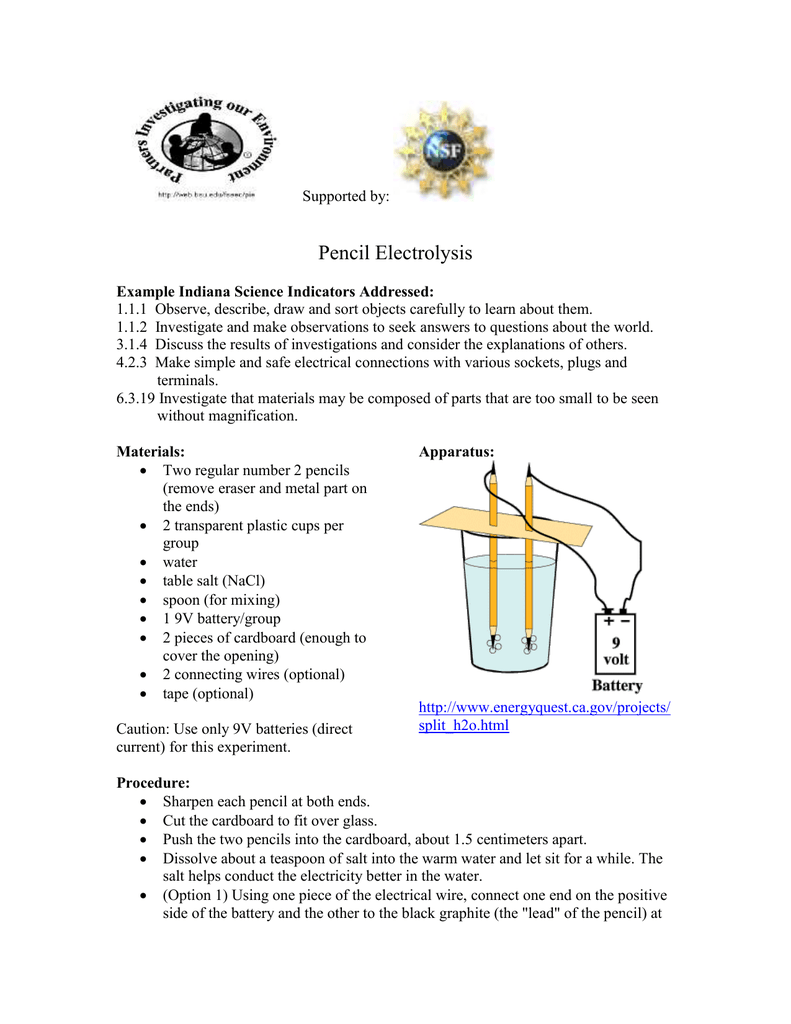










0 Response to "45 electrolysis of water lab answers"
Post a Comment