43 difference between ionic and molecular compounds
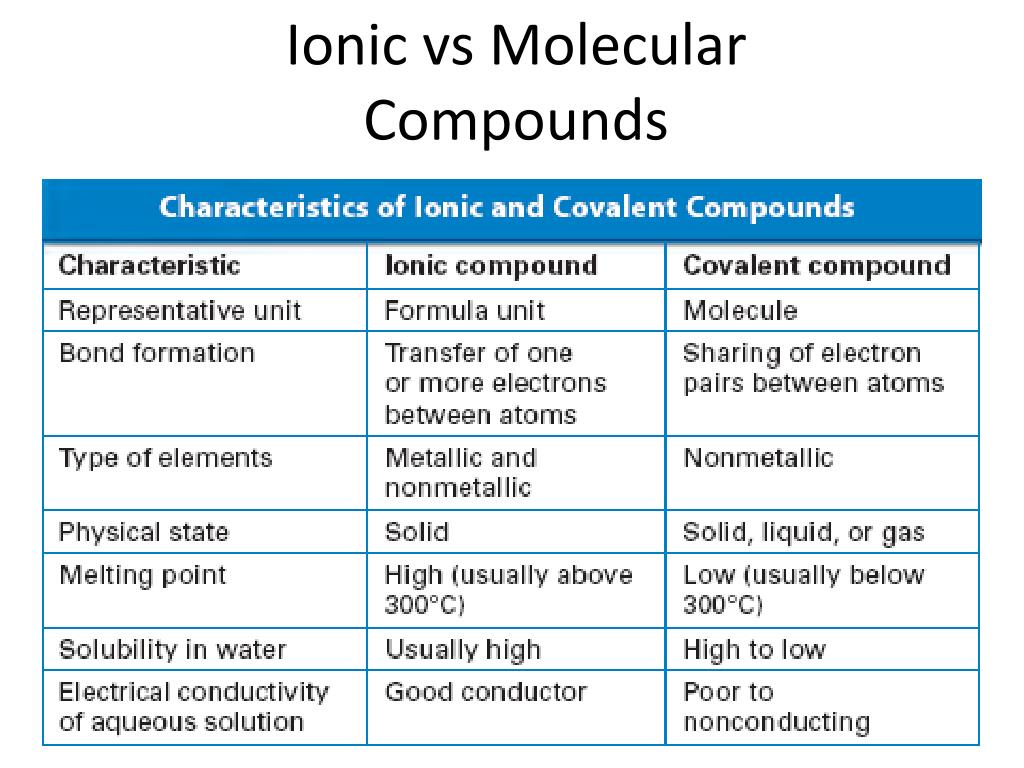
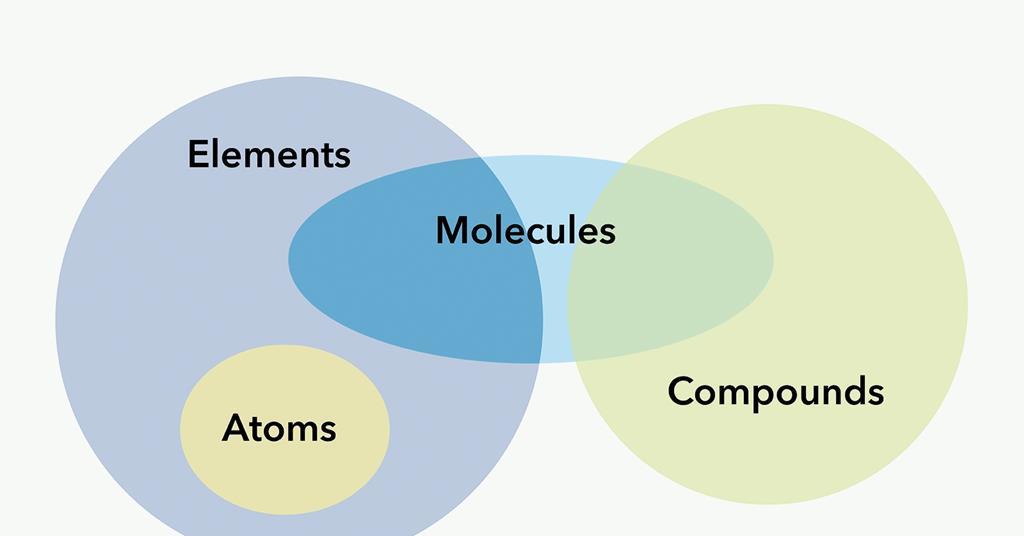
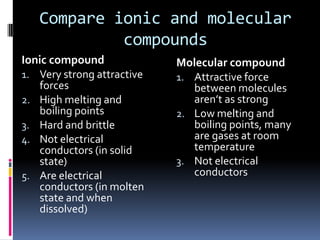
Ionic vs Molecular Compounds - Difference Between Ionic ... A molecular compound only requires low melting and boiling temperatures. It is not a good conductor of electricity. Main Differences Between Ionic vs Molecular Compounds. In showing the difference between molecular and ionic compounds, here is a tabular representation of their most important variations. Difference Between Ionic Compounds and Molecular Compounds ... Molecular Compounds are formed when two non-metals chemically combine, on the other hand, Ionic Compounds are formed between metal and non-mental. Molecular Compounds are formed due to covalent bond between elements, while Ionic Compound is formed due to electrostatic force of attraction known as Ionic bond. Molecular Compounds have low melting ...
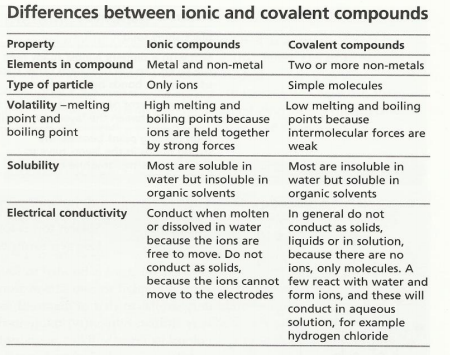
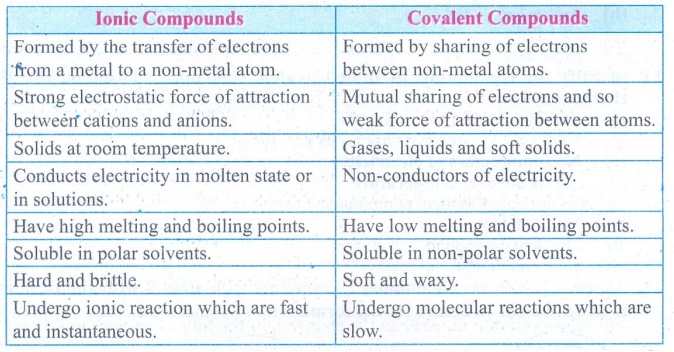
Difference Between Ionic and Covalent Compounds | Compare ... Key Difference - Ionic vs Covalent Compounds Many differences can be noted between ionic and covalent compounds based on their macroscopic properties such as solubility in water, electrical conductivity, melting points and boiling points.The main reason for these differences is the difference in their bonding pattern.

Difference between ionic and molecular compounds
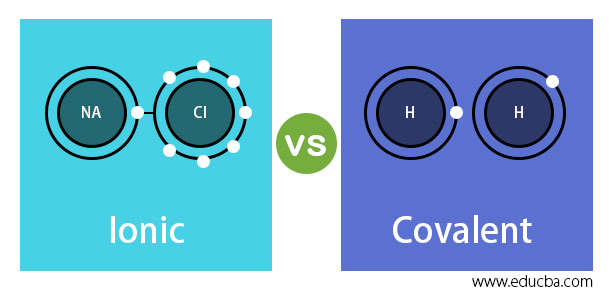
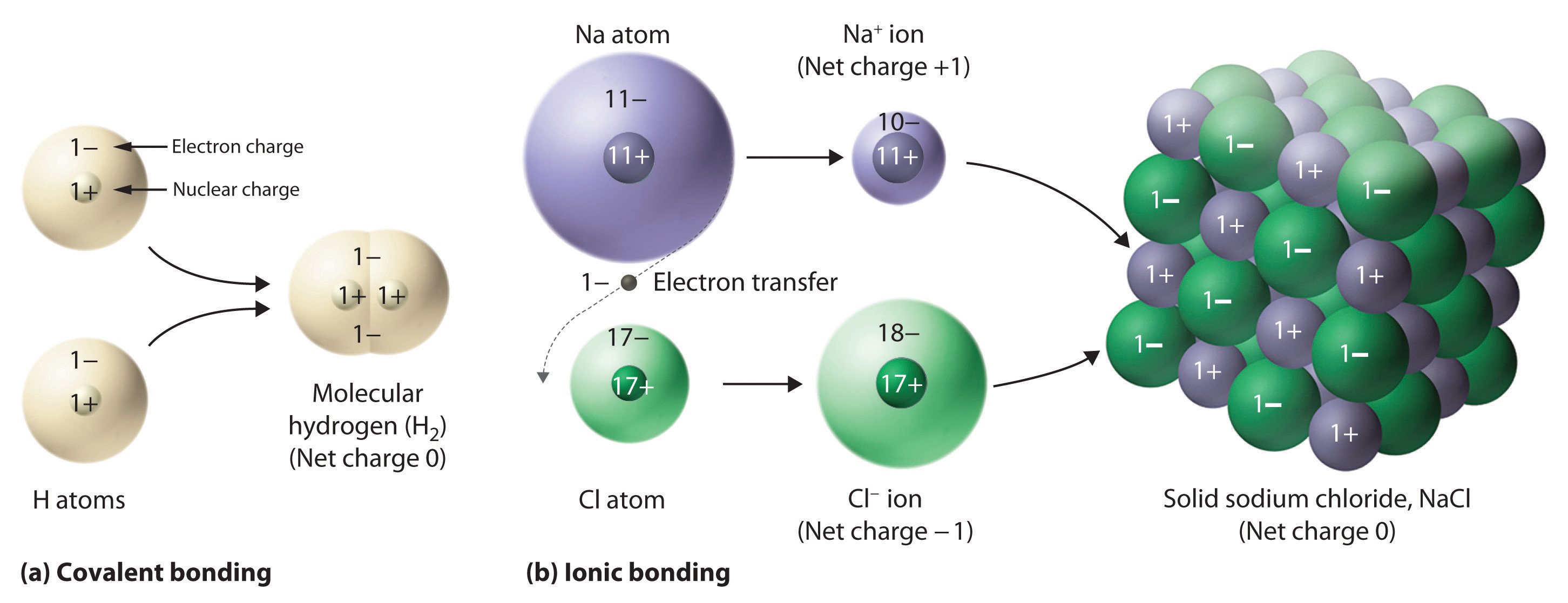
What Is the Difference Between Ionic and Molecular Compounds? Covalent compounds tend to burn easily, while ionic compounds do not. In contrast to ionic compounds, they are not water-soluble. Another distinction between the two types of molecules is that covalent compounds cannot conduct electricity, but ionic compounds generally can. What's the difference between an ionic and molecular ... Molecular compounds are formed between two non-metals while ionic compounds are formed between metals and non-metals. Are ionic or covalent bonds more common? Another way atoms can become more stable is by sharing electrons (rather than fully gaining or losing them), thus forming covalent bonds. PDF List the four major differences between ionic and covalent ... List the four major differences between ionic and covalent compounds. 1. Ionic bonds result from transfer of electrons, whereas covalent bonds are formed by sharing. 2. Ionic bonds are electrostatic in nature, resulting from that attraction of positive and negative ions that result from the electron transfer process; charge separation between ...
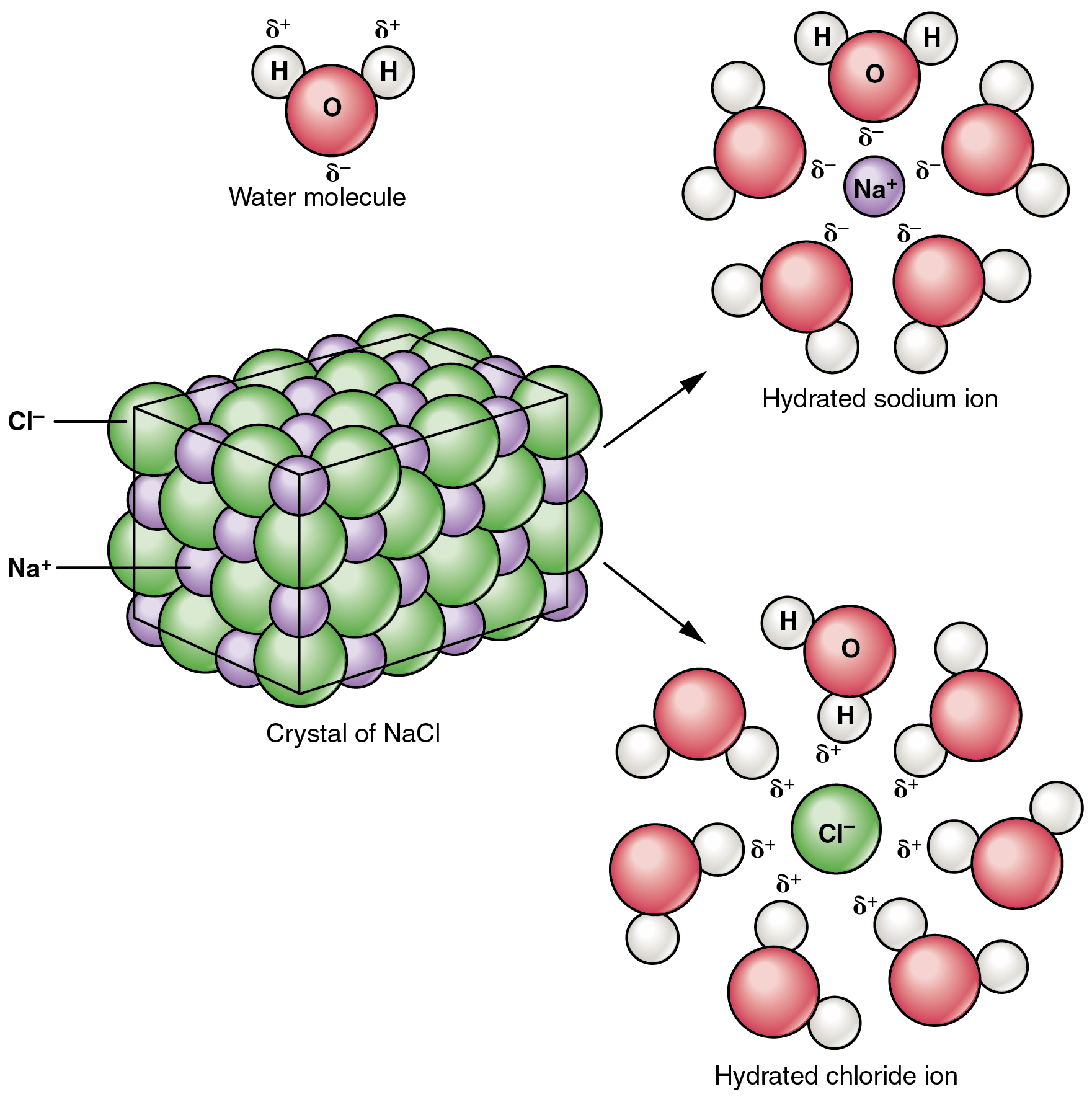
Difference between ionic and molecular compounds. Difference Between Ionic and Molecular Compound ... Difference Between Ionic and Molecular Compound Ionic vs Molecular Compound Molecular compounds are atoms linked together by sharing electrons. Basically they bind together in electrically neutral particles called molecules. Some molecular compounds are very simple. The very examples of these are diatomic molecules, which only consists of two atoms. Difference between Ionic and Molecular Compound ... The ionic and molecular compounds are two types of compounds that are present in the solid state. The difference between them is that the ions present in the ionic compounds are held together by electrostatic forces of attraction. The compounds are formed by the combination of metal and non-metal atoms. DIFFERENCES BETWEEN IONIC AND COVALENT COMPOUNDS - Quizlet Start studying DIFFERENCES BETWEEN IONIC AND COVALENT COMPOUNDS. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Ionic Compounds Vs. Molecular Compounds: What You Need to ... Differences between Ionic and Molecular Compounds. An ionic compound is formed by the reaction of a metal with a non-metal, whereas a molecular compound is usually formed by the reaction of two or more non-metals. In ionic compounds, the ions are held together due to electrical attraction, whereas, in molecular compounds, the atoms are held ...
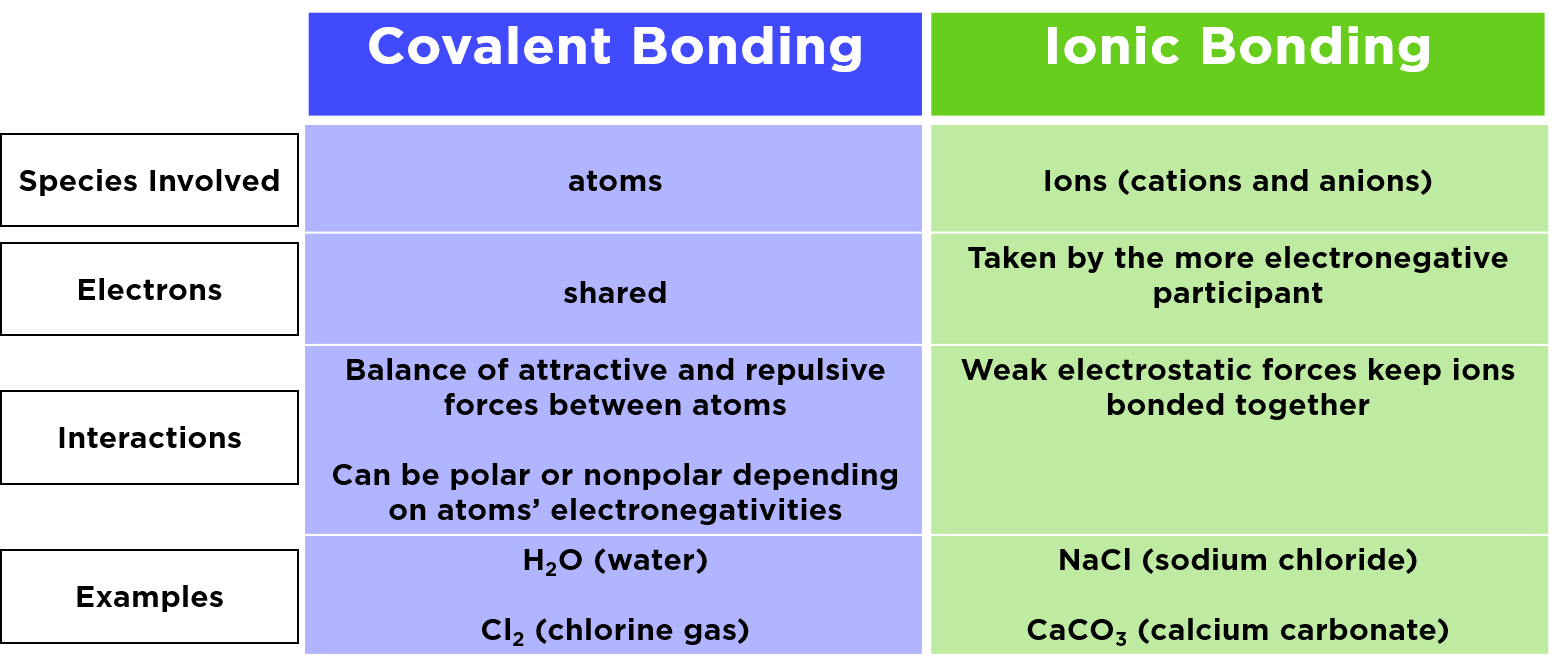
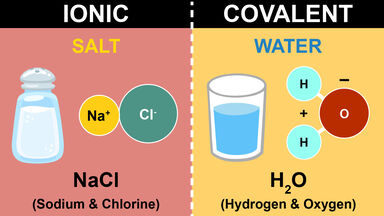
Difference Between Ionic, Covalent and Metallic bonds with ... Difference Between Ionic, Covalent and Metallic bonds The attractive force which holds together the atoms or group of atoms in a chemical species is known as a chemical bond. The definition of the chemical bond as a shared electron pair could be extended to describe the dative bond and the elaboration of Lewis acid/base interactions. Difference Between Ionic and Molecular Compounds | Compare ... The key difference between ionic and molecular compounds is that the ionic compounds have electrostatic attraction forces between cations and anions whereas the molecular compounds have only covalent chemical bonds between the atoms.. Chemical elements can join with each other to form chemical compounds. The elements bind together via chemical bonds that have ionic or covalent characteristics. Kami Export - Halyoteab Tsegai - Differences Between Ionic ... View Kami Export - Halyoteab Tsegai - Differences Between Ionic and Covalent Compounds Lab.pdf from PHIL 2030 at East Tennessee State University. Procedure 1. Put on your goggles and apron. 2. Clean what is the difference between a molecule and a compound ... Molecules of compounds have atoms of two or more different elements.For example, water (H 2 O) has three atoms, two hydrogen (H) atoms and one oxygen (O) atom.. Is molecule a compound? Compounds can be classified as ionic or covalent.Molecules are the simplest unit of a covalent compound, and molecules can be represented in many different ways.
What are the similarities and differences between ionic ... The difference between ionic and covalent compounds can be confusing. A basic definition of an ionic compound is that they are molecules that consist of charged ions . On the other hand, covalent compounds are non-metals which are bound together, and consist of two electrons that are shared between two atoms. Difference between ionic and molecular compounds - LORECENTRAL Main difference between ionic and molecular. The ionic compounds are linked by ionic bonds, while the molecular compounds are covalently bonded. An ionic bond is formed between two oppositely charged ions by means of electrostatic attraction. By Coulomb's law, the force of an electrostatic attraction is based on the proximity of atoms and the ... The Difference Between Ionic Compounds and Covalent ... Ionic Compounds vs Covalent Compounds. The main difference between Ionic Compounds and Covalent Compounds is their formation. The Ionic Compound is formed when there is a big difference in the electronegativity of the atoms, where the less electronegative atom loses an electron while the other gains it. Ionic vs. Molecular - YouTube To see all my Chemistry videos, check outhttp://socratic.org/chemistryHow can you tell the difference between compounds that are ionic and molecular (also kn...
Difference Between Ionic and Molecular Compounds Ionic compounds are made of ionic bonds, and molecular compounds are made of covalent bonds. Ionic bonds occur between two species which are electrostatically attracted towards each other, whereas covalent bonds from through the sharing of electrons between their outer shells. This is the main difference between ionic and molecular compounds.
What is the difference between ionic bond and ionic compounds? The only pure covalent bonds occur between identical atoms. Also Know, which is an ionic compound? In chemistry, an ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding. The compound is neutral overall, but consists of positively charged ions called cations and negatively charged ions ...
Difference Between Ionic Compounds and Molecular Compounds ... Molecular Compounds are formed when two non-metals chemically combine, on the other hand, Ionic Compounds are formed between metal and non-mental. Molecular Compounds are formed due to covalent bond between elements, while Ionic Compound is formed due to electrostatic force of attraction known as Ionic bond.
PDF Differences between Ionic and Molecular compounds Differences between Ionic and Molecular compounds . Ionic compounds: Covalent or Molecular compounds: 1. Are formed by electrostatic attraction between cations (atoms that have lost e-) between atoms. A shared pair of e and anions (atoms that have gained e-).
Ionic vs Covalent Bonds - Understand the Difference Covalent Bonds. In a covalent bond, the atoms are bound by shared electrons. In a true covalent bond, the electronegativity values are the same (e.g., H 2, O 3), although in practice the electronegativity values just need to be close.If the electron is shared equally between the atoms forming a covalent bond, then the bond is said to be nonpolar.. Usually, an electron is more attracted to one ...
What are the similarities and differences between ionic ... Answer: Some similarities are that they are both made from atoms. Some difference are that the atoms bonding in ionic compounds have a difference of 1.7+ in electronegativity, while molecular compounds have a difference in electronegativity of below 1.7. Ionic compounds can conduct electricity(wh...
Difference Between Ionic Compounds and Molecular Compounds ... The main difference between Ionic and Molecular Compounds is that the electrons of atoms in ionic compounds are transferred between the elements because of the presence of a difference in electronegativity. However, in the case of molecular compounds, the electrons are only shared but not transferred. To understand the difference better, you ...
Difference Between Ionic and Covalent Compounds Ionic bonds occur between two species which are electrostatically attracted towards each other, whereas covalent atoms bond covalently through the sharing of electrons between their outer shells. This is the main difference between Ionic and Covalent Compounds. In general, metallic elements tend to form ionic compounds, and non-metallic ...
Write 5 points of difference between ionic and covalent ... Suggest Corrections53. Q. (a) What are ionic compounds and covalent compounds? Give the name and formula of one ionic compound and one covalent compound. (b) Write the major points of difference between ionic compounds and covalent compounds. State differences between the properties of ionic compounds and covalent compounds.
Solved What is the difference between an ionic compound ... In ionic compounds the atoms (or group of atoms) exits as ions and are held together by covalent bonds. In molecular compounds the atoms are held together by electrostatic forces. In Ionic compounds the atoms (or group of atoms) exist an ions and are held together by electrostatic; Question: What is the difference between an ionic compound and ...
PDF List the four major differences between ionic and covalent ... List the four major differences between ionic and covalent compounds. 1. Ionic bonds result from transfer of electrons, whereas covalent bonds are formed by sharing. 2. Ionic bonds are electrostatic in nature, resulting from that attraction of positive and negative ions that result from the electron transfer process; charge separation between ...
What's the difference between an ionic and molecular ... Molecular compounds are formed between two non-metals while ionic compounds are formed between metals and non-metals. Are ionic or covalent bonds more common? Another way atoms can become more stable is by sharing electrons (rather than fully gaining or losing them), thus forming covalent bonds.
What Is the Difference Between Ionic and Molecular Compounds? Covalent compounds tend to burn easily, while ionic compounds do not. In contrast to ionic compounds, they are not water-soluble. Another distinction between the two types of molecules is that covalent compounds cannot conduct electricity, but ionic compounds generally can.











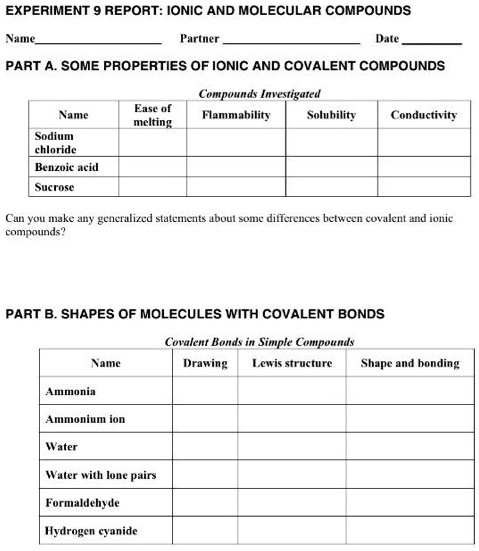

/covalent-bond-58de6d0b3df78c51627d1783.jpg)

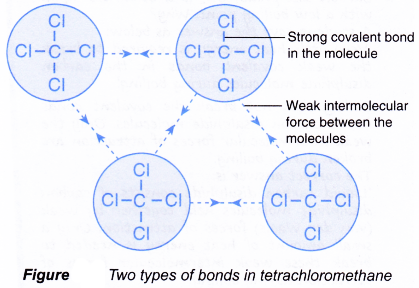
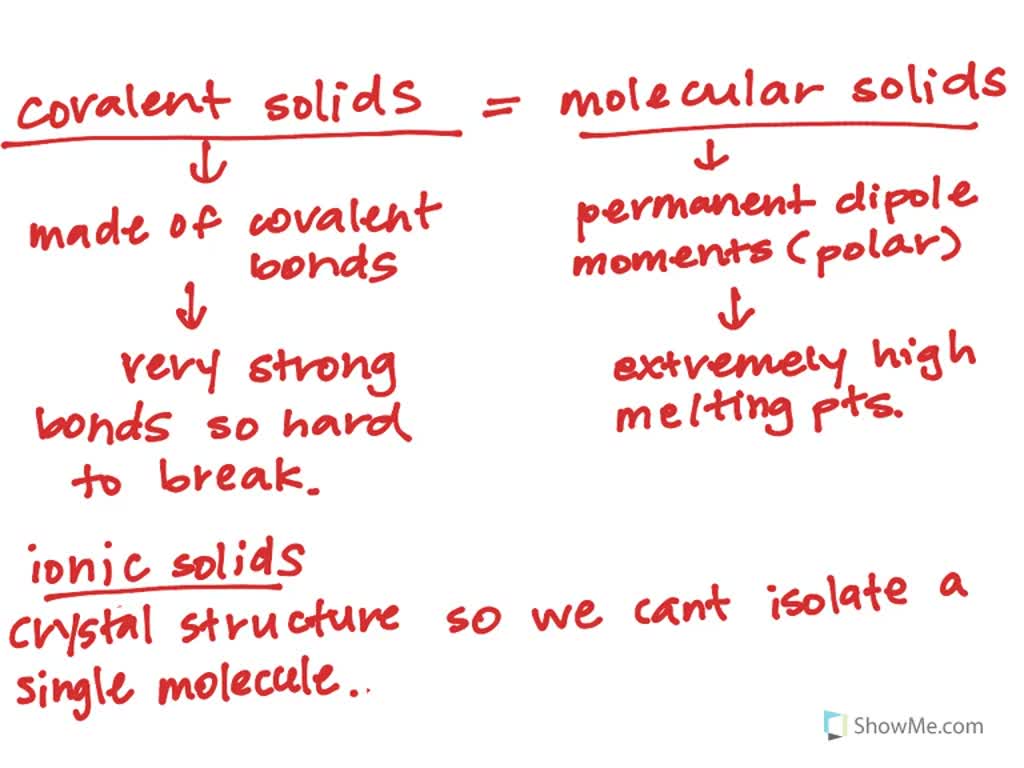
![Solved] 5 differences between ionic compound and covalent ...](https://hi-static.z-dn.net/files/d7e/e1076b848c7d9e67da705f00fc2d26bd.jpg)




0 Response to "43 difference between ionic and molecular compounds"
Post a Comment