40 why does alka seltzer dissolve in water
How does Alka-Seltzer Dissolves In Water? - Answers Alka-Seltzer contains various soluable compounds, such as asperin and citric acid, combined in a tablet form with bicarbonate of soda. The bicarbonate of soda reacts when it is in water, giving off... Why do Alka Seltzer tablets fizz in water? Alka Seltzer tablets easily dissolve in water. Water releases hydrogen ions that can react with the base inside the Alka Seltzer tablet, sodium bicarbonate. When the sodium bicarbonate reacts with the hydrogen ions, carbon dioxide forms, which causes the bubbles you see when you drop an Alka Seltzer tablet in water.
Pressure vs Rate of a Chemical Reaction | Alka-Seltzer STEP 1. Fill a 16 x 150 mm test tube 1/2 full of water. The water should be at about room temperature. STEP 2. Break an Alka-Seltzer tablet in half and drop the pieces into the test tube. STEP 3. Measure the time required for the reaction between the Alka-Seltzer and water to be completed. Record the time.

Why does alka seltzer dissolve in water
Alka Seltzer and Water Chemical Reaction - Study.com However, in the Alka-Seltzer, the two chemicals are in a solid form, they can't react because the molecules don't move. When they enter water, the chemicals are released and can react. In... Does Alka-Seltzer Dissolve In Water? Water releases hydrogen ions that can react with the base inside the Alka Seltzer tablet, sodium bicarbonate. When the sodium bicarbonate reacts with the hydrogen ions, carbon dioxide forms, which causes the bubbles you see when you drop an Alka Seltzer tablet in water. Table of Contents What happens when you put an Alka Seltzer in water? Why does Alka Seltzer dissolve faster in water than soda ... Why does Alka Seltzer dissolve faster in water than soda? The Alka-Seltzer tablet dissolved the quickest in the water because water is the liquid that the Alka-Seltzer tablet was designed to dissolve in. Also, Alka-Seltzer tablets dissolve quicker in larger amounts of liquid compared to smaller amounts of liquid.
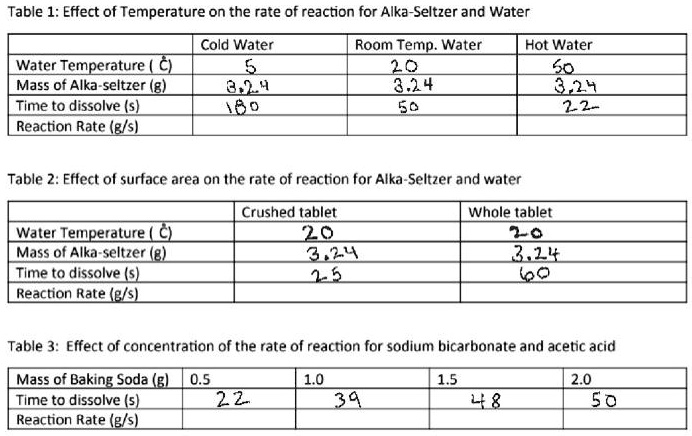
Why does alka seltzer dissolve in water. How to use alka seltzer - virtualpsychcentre.com The whole tablets dropped into cold water proved to be the slowest chemical reaction.As a result of the experiment, the hot water caused the Alka-Seltzer tablets to dissolve about 2 times quicker than water at lukewarm temperatures, and about 3 times faster than water at a cold temperature. Why does a alka-seltzer tablet dissolve slower in cold ... Alka-Seltzer tablets dissolve faster in hot water rather than cold because the more heat something has the more energy the atoms or molecules in the object get. so in hot water the molecules ... Alka Seltzer & Temperature Experiment - Video & Lesson ... Alka Seltzer tablets easily dissolve in water. Water releases hydrogen ions that can react with the base inside the Alka Seltzer tablet, sodium bicarbonate. When the sodium bicarbonate reacts with... Does Alka Seltzer React To Cooking Oil? - mcginnis-sisters.com Does Alka-Seltzer React With Oil? Alka-Seltzer does not dissolve in the slightest when used in oil or water. The oil and the water do not mix. Due to oil's lighter nature, when it is added to water, it sits on top as the water drips. Food coloring does not dissolve in oil, and its color differs from oil because it has a heavier base.
Does Alka Seltzer react with vinegar? - AskingLot.com In Alka-Seltzer, the citric acid mixes with the base, bicarbonate, to form carbon dioxide bubbles. Similarly, why does Alka Seltzer dissolve faster in vinegar? Based on the data gathered, the less concentrated the vinegar solution is, the faster the Alka-Seltzer tablet dissolves. As the vinegar concentrated decreased, the water concentration ... The Alka Seltzer Reaction - Middle School Chemical ... The reaction in this activity involves using sodium bicarbonate and citric acid to produce water and carbon dioxide. Reaction: HCO 3- (aq) + H + (aq) → H 2 O (l) + CO 2 (g) The tablets contain sodium bicarbonate (NaHCO 3) and citric acid. When the tablet is dissolved in water, bicarbonate (HCO 3-) and hydrogen ions (H +) are formed. PDF The Alka-Seltzer Lab! Station 1: Will Alka-Seltzer tablets dissolve faster or slower in carbonated water compared to regular water? Station 2: Will a broken Alka-Seltzer tablet dissolve faster or slower than the whole tablet in carbonated water? Station 3: Does an Alka-Seltzer tablet dissolve differently in a salt water? Station 4: Will Alka-Seltzer tablets dissolve faster or slower in hot water compared to room ... Why does hot water make things dissolve faster ... Why does hot water dissolve Alka Seltzer faster? The second thing learned is the reason why the Alka-Seltzer dissolves in hot water faster than in cold water. The reason is because higher temperatures have higher kinetic molecular energy. This causes the Alka-Seltzer to react faster.
What are the products when Alka Seltzer dissolves in water ... When water is added, the sodium bicarbonate and citric acid are dissolved, forming aqueous ions which react to yield carbon dioxide, water, and the sodium salt of citric acid (sodium citrate). The pH of the final solution is sufficiently basic to act as a remedy for acid stomach and the aspirin simply dissolves in the water, so it can be consumed more easily so that it can fight headaches or similar types of pain. How long does alka seltzer take to work - Meanings.co In short: the medicine dissolves fast and goes to work instantly, neutralizing acid on contact.Because the medicines in Alka-Seltzer effervescents are in solution prior to ingestion, they do not require time to dissolve in the stomach like similar active ingredients taken in a tablet form. Does Alka-Seltzer turn into gas? - idswater.com Does Alka-Seltzer dissolve faster in hot or cold water? The whole tablets dropped into cold water proved to be the slowest chemical reaction. As a result of the experiment, the hot water caused the Alka-Seltzer tablets to dissolve about 2 times quicker than water at lukewarm temperatures, and about 3 times faster than water at a cold temperature. Why does Alka-Seltzer dissolve slower in boiling water ... Answer (1 of 2): the solubility of carbonates decreases as the temperature increases. This is also why carbonate scale tends to accumulate in the hot water side of household plumbing systems when the water is "hard" or high in dissolved carbonates - usually from groundwater. Why does the solubil...
Why does Alka Seltzer fizz? - HowStuffWorks Why does Alka Seltzer fizz? The fizzing you see when you drop an Alka-Seltzer tablet in water is the same sort of fizzing that you see from baking powder. If you look at the Question of the Day on baking powder, you will find that the baking powder reaction is caused by an acid reacting with baking soda (sodium bicarbonate).
Effect of Temperature on Rate of Reaction | Alka-Seltzer® Part A. Hot Water. Run water from the hot tap until it is as hot as possible. Fill a clear glass with exactly 8 oz./240 mL of hot water. Use the thermometer to take the temperature and record it on your data sheet. Remove 1 Alka-Seltzer tablet from its package. Drop it into water. Measure the time required for tablet to fully dissolve.

Alka-Seltzer Plus Severe Cold Medicine, Sparkling Original Powerfast Fizz Effervescent Tablets, 36 Count
Does Alka-Seltzer dissolve faster in more or less water ... As a result of the experiment, the hot water caused the Alka-Seltzer tablets to dissolve about 2 times quicker than water at lukewarm temperatures, and about 3 times faster than water at a cold temperature. What happens when you put an Alka Seltzer tablet in water?
Why Does Alka Seltzer Fizz In Water So Well ... Does Alka-Seltzer Dissolve Faster In More Water? In comparison, the process of slowly boiling the tablets found the process to be slower. At lukewarm temperatures, the Alka-Seltzer tablets dissolved about twice as readily as at a cold temperature, and they dissolved about 3 times as quickly in a hot one.
Carbonation Countdown: The Effect of Temperature on ... To take the tablets, they're fully dissolved in water, where they famously undergo a chemical reaction that produces lots of carbon dioxide bubbles—or fizz. Why is this? As the tablets dissolve,...
Alka-Seltzer Science: The Effect of Temperature on ... Have you ever wondered why bubbles form when an Alka-Seltzer tablet is put in water? If you've ever tried it, you've seen that the tablet fizzes furiously when dropped into water. The moment the tablet starts dissolving, a chemical reaction occurs that releases carbon dioxide gas. This is what the bubbles are.
What Makes Alka Seltzer Dissolve Faster, Background Research After the Alka-Seltzer tablet was added to the warm water the tablet should have quickly dissolved, taking some 20 come 30 secs to execute so, relying on the specific temperature. ~ the tablet computer was included to the ice-cold water that should have actually taken much longer to dissolve, with most of the tablet computer disappearing after ...
Does Alka seltzer dissolve faster in hot or cold water by ... My results are that the alka seltzer dissolves faster in hot water because all the trials the time for hot water is faster.The results do support my hypothesis.
What makes a tablet dissolve faster ... Do tablets dissolve faster in hot or cold water? The whole tablets dropped into cold water proved to be the slowest chemical reaction. As a result of the experiment, the hot water caused the Alka-Seltzer tablets to dissolve about 2 times quicker than water at lukewarm temperatures, and about 3 times faster than water at a cold temperature.
Plop, Plop, Fizz Fast: The Effect of Temperature on ... Alka-Seltzer® tablets fizzle furiously when dropped into water. The moment the tablet starts dissolving, a chemical reaction occurs that releases carbon dioxide gas. In this science project, you can even measure how long and loudly your tablet fizzes using a smartphone equipped with a sensor app.
Why does Alka Seltzer dissolve faster in water than soda ... Why does Alka Seltzer dissolve faster in water than soda? The Alka-Seltzer tablet dissolved the quickest in the water because water is the liquid that the Alka-Seltzer tablet was designed to dissolve in. Also, Alka-Seltzer tablets dissolve quicker in larger amounts of liquid compared to smaller amounts of liquid.
Does Alka-Seltzer Dissolve In Water? Water releases hydrogen ions that can react with the base inside the Alka Seltzer tablet, sodium bicarbonate. When the sodium bicarbonate reacts with the hydrogen ions, carbon dioxide forms, which causes the bubbles you see when you drop an Alka Seltzer tablet in water. Table of Contents What happens when you put an Alka Seltzer in water?
Alka Seltzer and Water Chemical Reaction - Study.com However, in the Alka-Seltzer, the two chemicals are in a solid form, they can't react because the molecules don't move. When they enter water, the chemicals are released and can react. In...


































0 Response to "40 why does alka seltzer dissolve in water"
Post a Comment