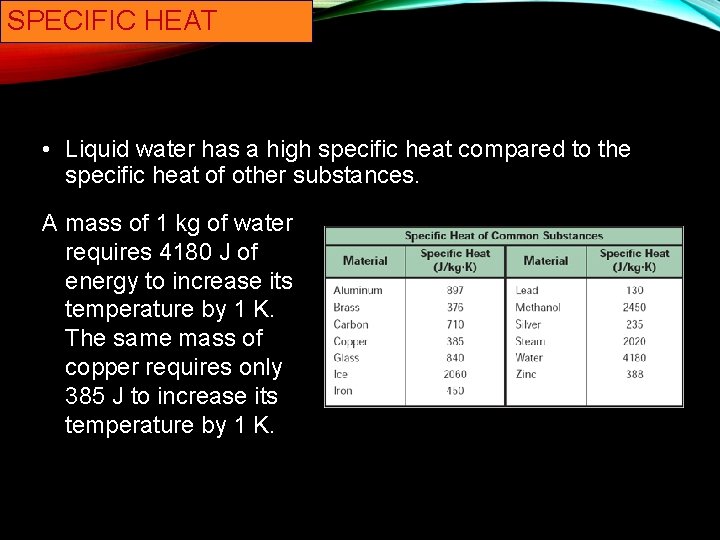
43 What Is The Specific Heat Of Liquid Water
Specific Heat of Liquids - an overview | ScienceDirect Topics The maximum temperature rise recommended for any fluid is 15°F (can be a bit higher at times for the average process condition) except when handling cold fluids or using a special pump designed to handle hot fluid, such as a boiler feed water pump of several manufacturers. Sign in to download full-size image Figure 5-41. Specific Heat of Water: Definition, Explanation and Formula The specific heat capacity (C p) of a liquid at normal temperature and pressure is approximately 4.2 J/g°C. This means that 1 gram of water requires 4.2 joules of energy to raise 1 degree Celsius. This C p number is fairly high. This is the specific heat of the water as a liquid (1 cal/g.deg) or the specific heat capacity of liquid water.
Specific Heat Capacity - an overview | ScienceDirect Topics Specific heat capacity, thermal expansion, heat conduction, thermal radiation, and thermoelectric force are all aspects of thermal performance. 5.2.3.1 Specific heat capacity Specific heat capacity is the energy required to increase temperature of material of a certain mass by 1°C, in the unit of J/(kg·K).

What is the specific heat of liquid water
Water - Wikipedia Along with oxidane, water is one of the two official names for the chemical compound H 2 O; it is also the liquid phase of H 2 O. The other two common states of matter of water are the solid phase, ice, and the gaseous phase, water vapor or steam.The addition or removal of heat can cause phase transitions: freezing (water to ice), melting (ice to water), vaporization (water to vapor ... What is the Enthalpy of liquid water? - Theburningofrome.com Water - Heat Capacity (Specific Heat) - Online calculator, figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to 360 °C (32-700 °F) - SI and Imperial units. What is the specific heat of water? | AnswersDrive Water has the highest specific heat capacity of any liquid. Specific heat is defined as the amount of heat one gram of a substance must absorb or lose to change its temperature by one degree Celsius. For water, this amount is one calorie, or 4.184 Joules.
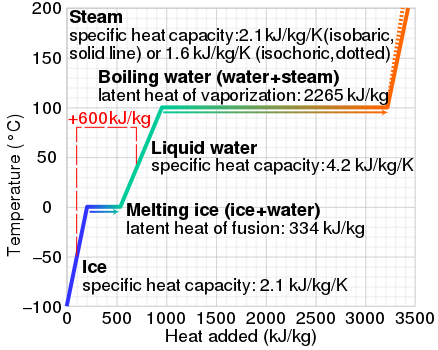
What is the specific heat of liquid water. [Solved] (a) What is the specific heat of liquid water? (b ... Figure 17-30 shows a temperature-versus-heat plot for 1.000 kg of water. Figure 17-30, (a) Calculate the heat corresponding to the points A, B, C, and D.(b) Calculate the slope of the line from point B to point C. Show that this slope is equal to 1/c, where c is the specific heat of liquid... What is the sensible heat of water? - FindAnyAnswer.com Sensible heat of water: It is defined as the quantity of heat absorbed by 1 kg ofwater when it is heated from 0°C (freezing point) to boiling point.It is also called total heat (orenthalpy) of water or liquid heat invariably. It is reckoned from 0°C where sensible heat is takenas zero. Click to see full answer Specific Heat Calculator Calculate specific heat as c = Q / (mΔT). In our example, it will be equal to c = -63,000 J / (5 kg * -3 K) = 4,200 J/ (kg·K). This is the typical heat capacity of water. If you have problems with the units, feel free to use our temperature conversion or weight conversion calculators. Why do liquids have higher specific heat capacity than ... Can liquid water be over 100 degrees? Liquid water can be hotter than 100 °C (212 °F) and colder than 0 °C (32 °F). Heating water above its boiling point without boiling is called superheating. If water is superheated, it can exceed its boiling point without boiling. To experience this, put a container of bottled water into a bowl of ice.
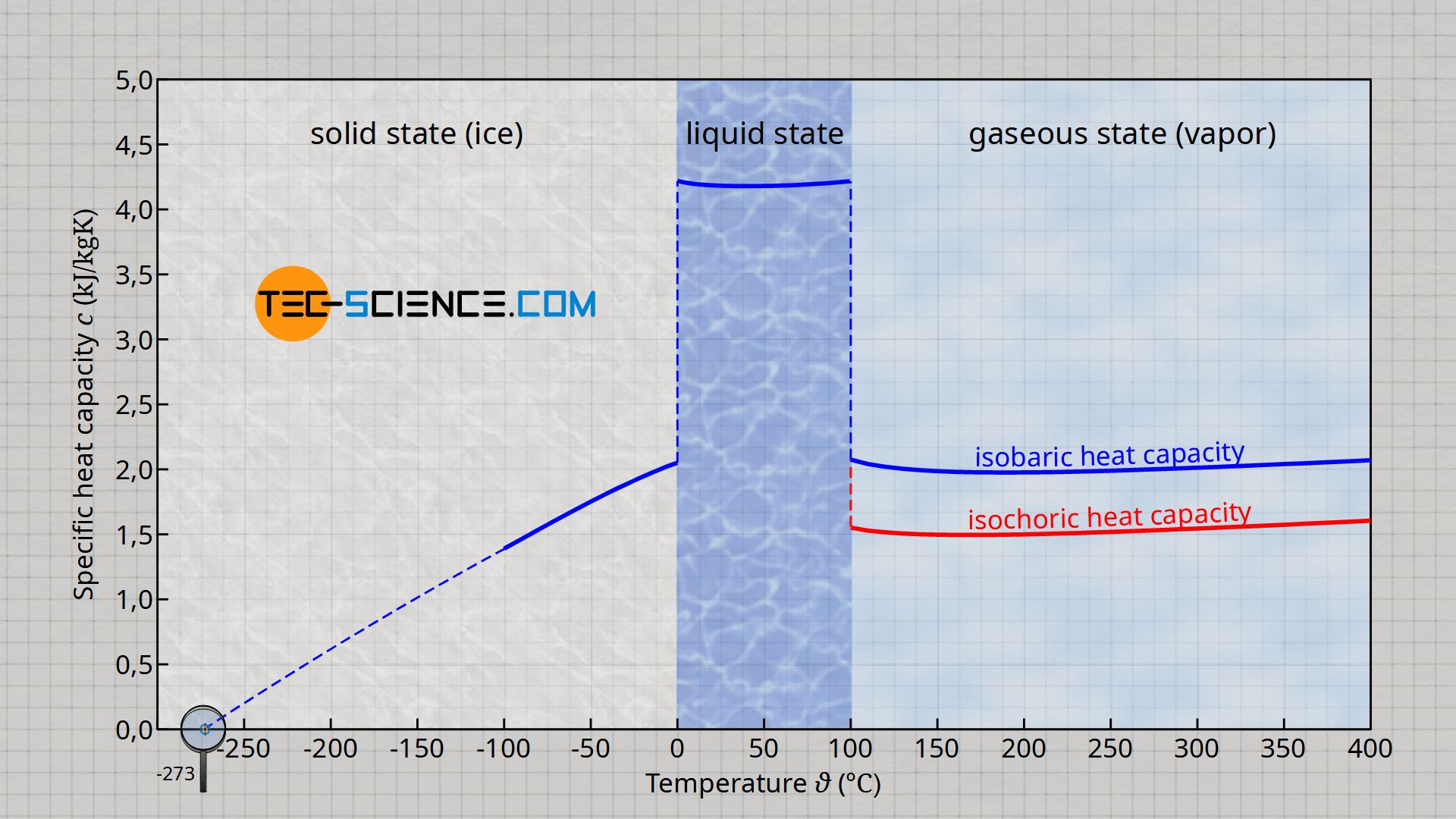
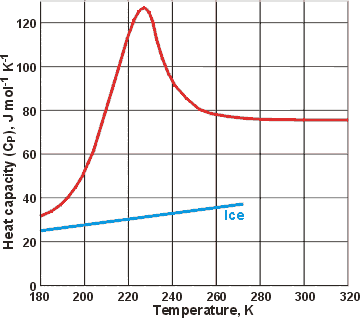
Specific Heat Calculator - Find Heat Capacity of Substances specific heat of water has one of the maximum specific heat values among common substances. It is about \ (4182 J/ (K kg) at 20 °C\). In case of ice it is only \ (2093 J/ (K kg)\). How to calculate specific heat (step-by-step)? With the support of specific heat formula calculation of specific heat is and easy process. What is the specific heat capacity of ice, water and steam ... Specific Heat of water, Ice and water vapour Water For liquid at room temperature and pressure, the value of specific heat capacity (C p) is approximately 4.187 kJ/kgK. Ice For ice 2.108 kJ/kgK. Water vapour For water vapor (steam) 1.996 kJ/kgK. Was this answer helpful? 3.5 (20) (27) (9) What Is The Molar Heat Capacity Of Liquid Water Finally, What is the specific heat capacity for liquid water?, Liquid water has one of the highest specific heats among common substances, about 4184 J·kg −1 ·K −1 at 20 °C; but that of ice just below 0 °C is only 2093 J·kg −1 ·K −1. The specific heats of iron, granite, and hydrogen gas are about 449, 790, and 14300 J·kg −1 ·K −1, respectively. Specific Heat Capacity: Definition, Molar Specific Heat ... Molar Specific Heat. The Molar specific heat of a solid or liquid of a material is the heat that you provide to raise the temperature of one mole of solid or liquid through 1K or 1° C. We represent it as C. Its unit is J mol-1K-1. So, to raise the temperature of µ moles of solid through ∆T, you would need an amount of heat equal to ∆Q=µ ...
What Is The Specific Heat Value Of Water? [Comprehensive ... The specific heat can be found by rearranging the formula: The specific heat of water is 4190 J/kg∙K. As said earlier, the specific heat is the ratio of the quantity of heat which is needed to increase the temperature of a given mass of any substance 10 C to the quantity needed to raise the temperature of an equal mass of water of 10 C.The ... What is the value of the specific heat capacity of liquid ... Answer (1 of 2): Specific heat water = 4.182 J/g °C Molar mass water = 18.0 g/mol specific heat capacity of liquid water in J/mol·°C: = 18g/mol * 4.182 J/g °C = 75.276 J/mol °C How thermally conductive is water? Water is one of the best choices for liquid cooling applications due to its high heat capacity and thermal conductivity. It is also compatible with copper, which is one of the best heat transfer materials to use for your fluid path. What Is the Molar Heat Capacity of Liquid Water? The molar heat capacity of liquid water is 75.348 J/mol K. It is calculated as the product of the specific heat capacity of liquid water and the molar mass of water. The specific heat capacity of liquid water is 4.186 J/gm K.
Transcribed image text : What is the specific heat of ... What is the molar heat capacity of liquid water? Express your answer using four significant figures. J/mol middot degree C What is the heat capacity of 180 g of liquid water? How many kJ of heat are needed to raise the temperature of 10.00 kg of liquid water from 24.4 degree C to 46.1 degree C? kJ. Feb 18 2022 11:16 AM. Solution.pdf.
Liquids and Fluids - Specific Heats - Engineering ToolBox 10 kg of water is heated from 20 oC to 100 oC - a temperature difference 80 oC (K). The heat required can be calculated as q = (4.19 kJ/kg K) (10 kg) (80 oC) = 3352 kJ Mixing Liquids and/or Solids - Final Temperatures Related Documents
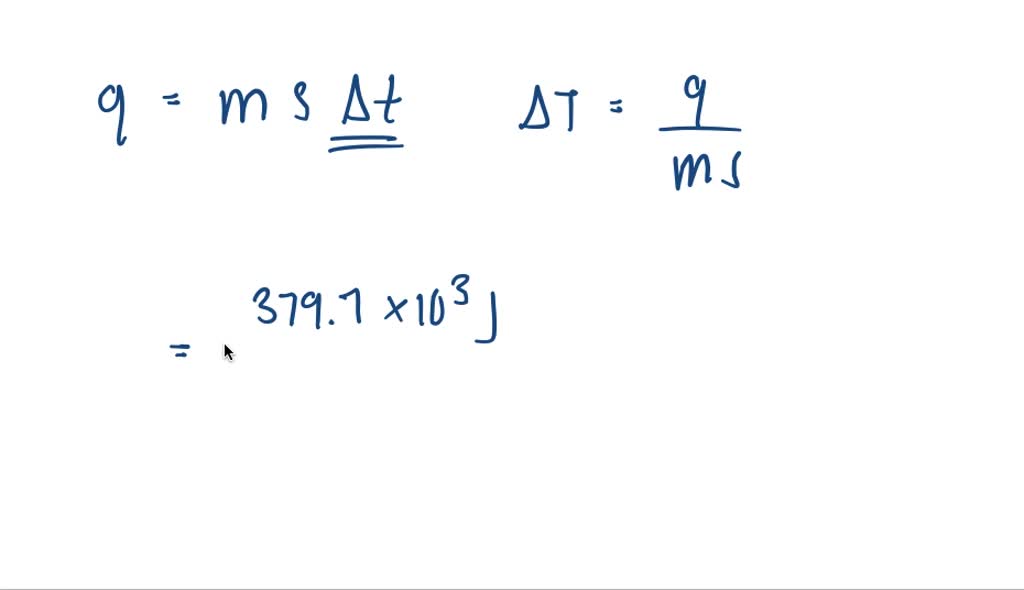
when steam condenses to liquid water 226 mathrmkj of heat is released per gram the heat from 168 mat
PDF Heat capacity of water - VaxaSoftware Heat capacity of liquid water from 0 °C to 100 °C Temp. Heat capacity Temp. Heat capacity Temp. Heat capacity °C K·kg kJ K·kg kcal °C K·kg kJ K·kg kcal °C K·kg kJ K·kg kcal 0 (ice) 1.960 0.468 34 4.178 0.999 68 4.189 1.001 0 4.217 1.008 35 4.178 0.999 69 4.189 1.001
Water - Specific Heat vs. Temperature - Engineering ToolBox Specific heat for liquid water at temperatures from 32 to 675 °F: Sponsored Links Engineering ToolBox - SketchUp Extension - Online 3D modeling!
Solved Part A What is the specific heat of liquid water ... 100% (7 ratings) Transcribed image text: Part A What is the specific heat of liquid water? Express your answer using four significant figures. 四? J/g.° C Submit My Answers Give Up Part B What is the molar heat capacity of liquid water? Express your answer using four significant figures. J/mol.° C Submit My Answers Give Up.
What Is the Specific Heat of Water? How Is It Special? Water has a specific heat capacity of 4182 J/kg°C. Because water is such an important and common substance, we even have a special way to identify the amount of energy it takes to raise one gram of water by one degree Celsius—a Calorie. This is different from the kind of calorie we talk about in food.
DOC Specific Heat Calculations - Horace Mann School The specific heat of liquid water is 4.18 J/g x oC. What is the mass of the sample of water? 84.3g. 850 calories of heat are applied to a 250 g sample of liquid water with an initial temperature of 13.0 oC. Find a) the change in temperature and b) the final temperature. ...
Specific Heat Capacity & Water - Formula & Detailed ... Specific Heat of Water For liquid at room temperature and pressure, the value of specific heat capacity (Cp) is approximately 4.2 J/g°C. This implies that it takes 4.2 joules of energy to raise 1 gram of water by 1 degree Celsius. This value for Cp is actually quite large.
The specific heat capacity of liquid water is 4.18 kJ/g C ... "239 J" First thing first, you mistyped the specific heat of water, which should be c_"water" = 4.18"J"/("g" ""^@"C") Now, a substance's specific heat tells you how much heat is required to increase the temperature of "1 g" of that substance by 1^@"C". In the case of water, you would need "4.18 J" to increase the temperature of "1 g" of water by 1^@"C". Notice that your sample of water has a ...
Specific Heat Capacity of Water | Earth 501: Contemporary ... The specific heat capacity (C p) of liquid water at room temperature and pressure is approximately 4.2 J/g°C. This means it takes 4.2 joules of energy to raise 1 gram (or 1 milliliter if you'd rather think of the equivalent volume of 1 gram of water) of water by 1 degree Celsius. This is actually quite large.
SOLVED:(a) What is the specific heat of liquid water? (b ... Hi there. We have a set of questions here centering around specific heat and heat capacity for water. So starting off with letter, a letter A is asking about the specific heat of liquid water. This is a constant. So we need to look that up and when we look it up, we see that it is 4.184 Jules program Degrees Celsius or be jewels program Kelvin because they change in a Kelvin degree and the ...
Specific Heat Capacity and Water | U.S. Geological Survey Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree Celsius (°C). Water has a high specific heat, meaning it takes more energy to increase the temperature of water compared to other substances. This is why water is valuable to industries and in your car's radiator as a coolant.
What is the specific heat of water? | AnswersDrive Water has the highest specific heat capacity of any liquid. Specific heat is defined as the amount of heat one gram of a substance must absorb or lose to change its temperature by one degree Celsius. For water, this amount is one calorie, or 4.184 Joules.
What is the Enthalpy of liquid water? - Theburningofrome.com Water - Heat Capacity (Specific Heat) - Online calculator, figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to 360 °C (32-700 °F) - SI and Imperial units.
Water - Wikipedia Along with oxidane, water is one of the two official names for the chemical compound H 2 O; it is also the liquid phase of H 2 O. The other two common states of matter of water are the solid phase, ice, and the gaseous phase, water vapor or steam.The addition or removal of heat can cause phase transitions: freezing (water to ice), melting (ice to water), vaporization (water to vapor ...





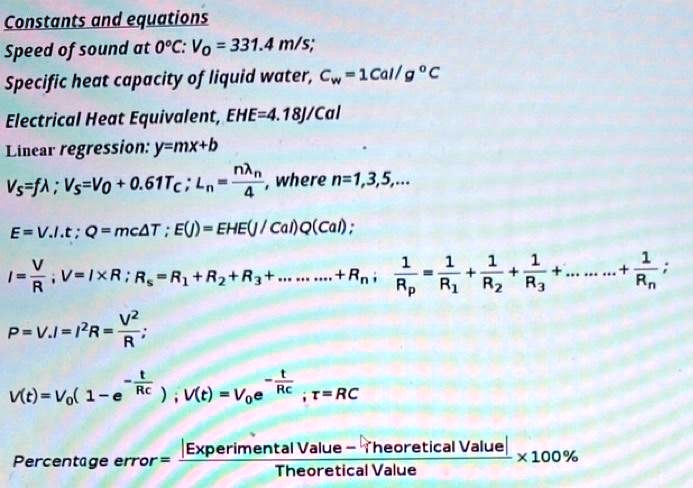



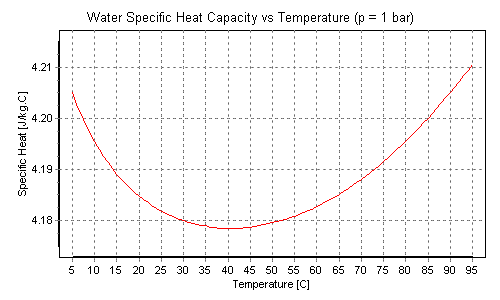

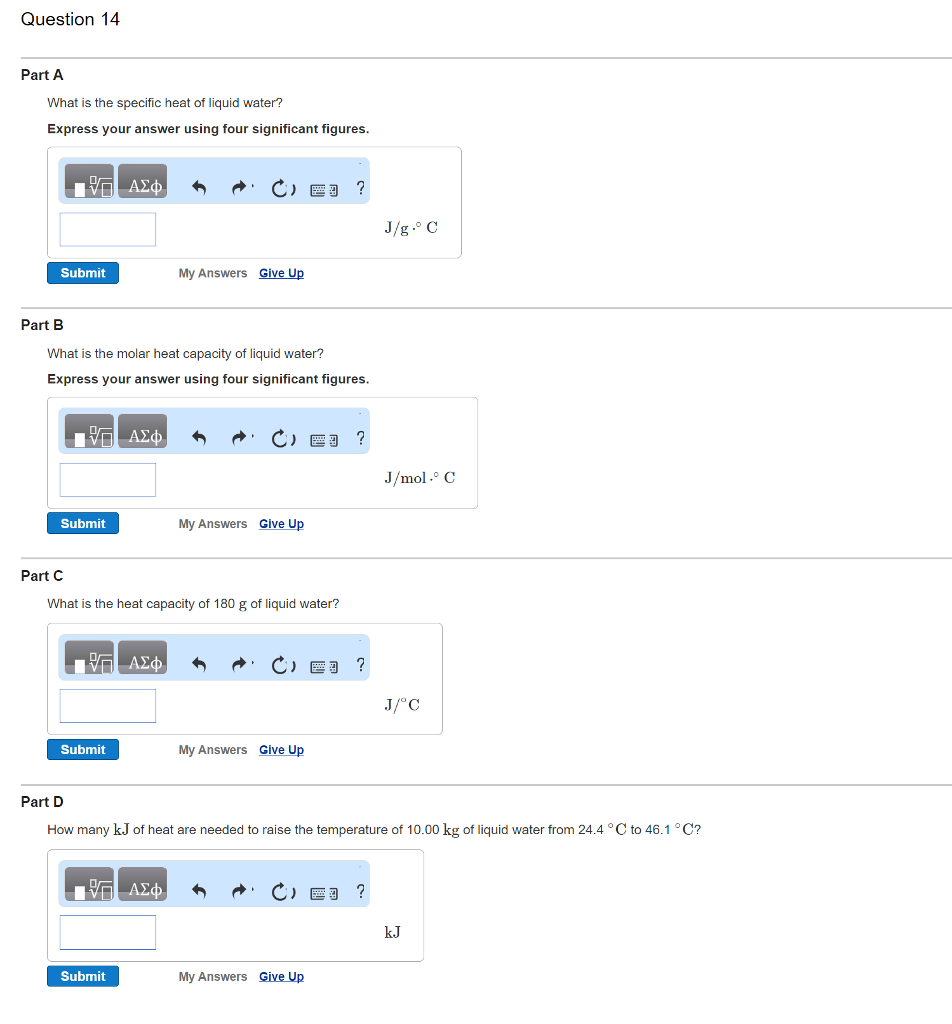








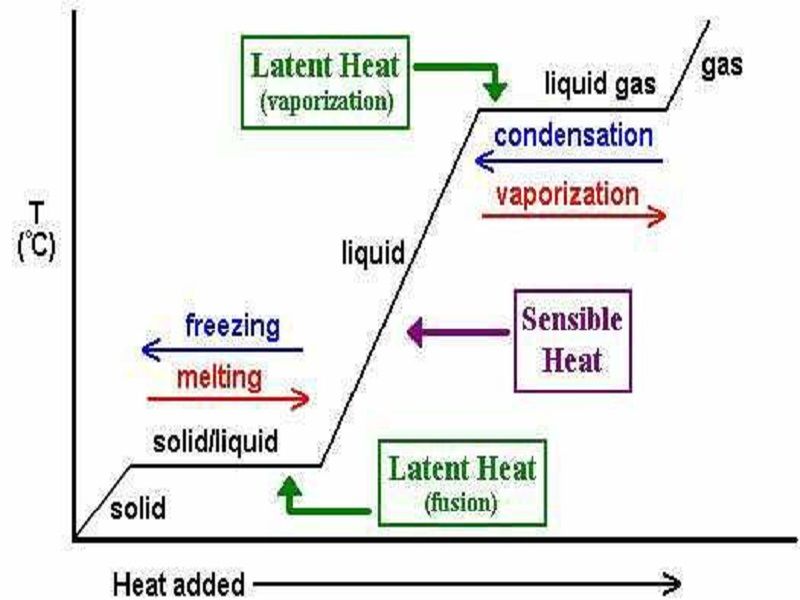
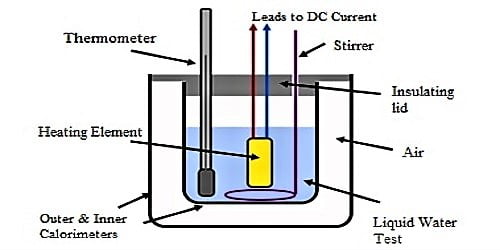




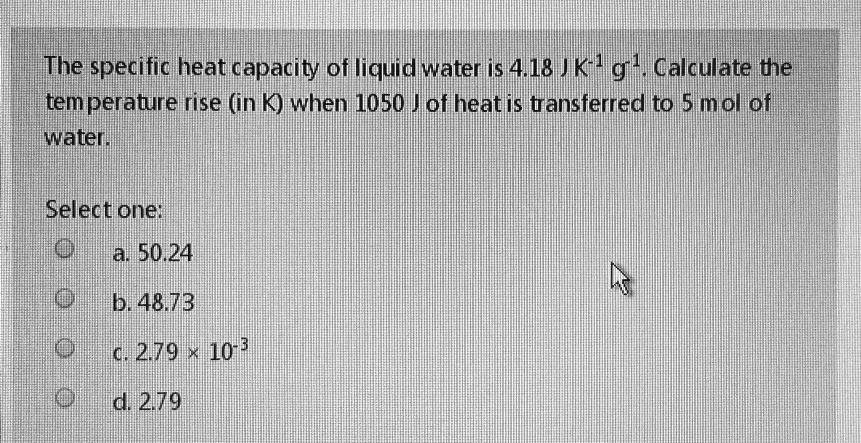


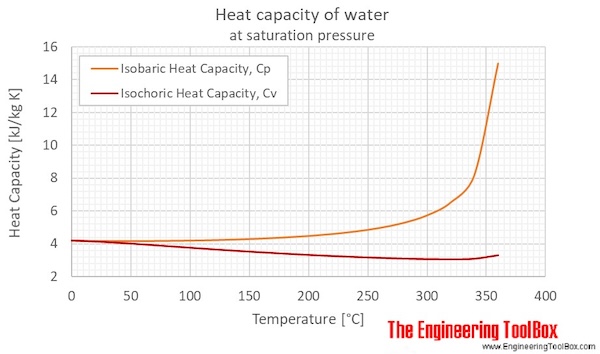
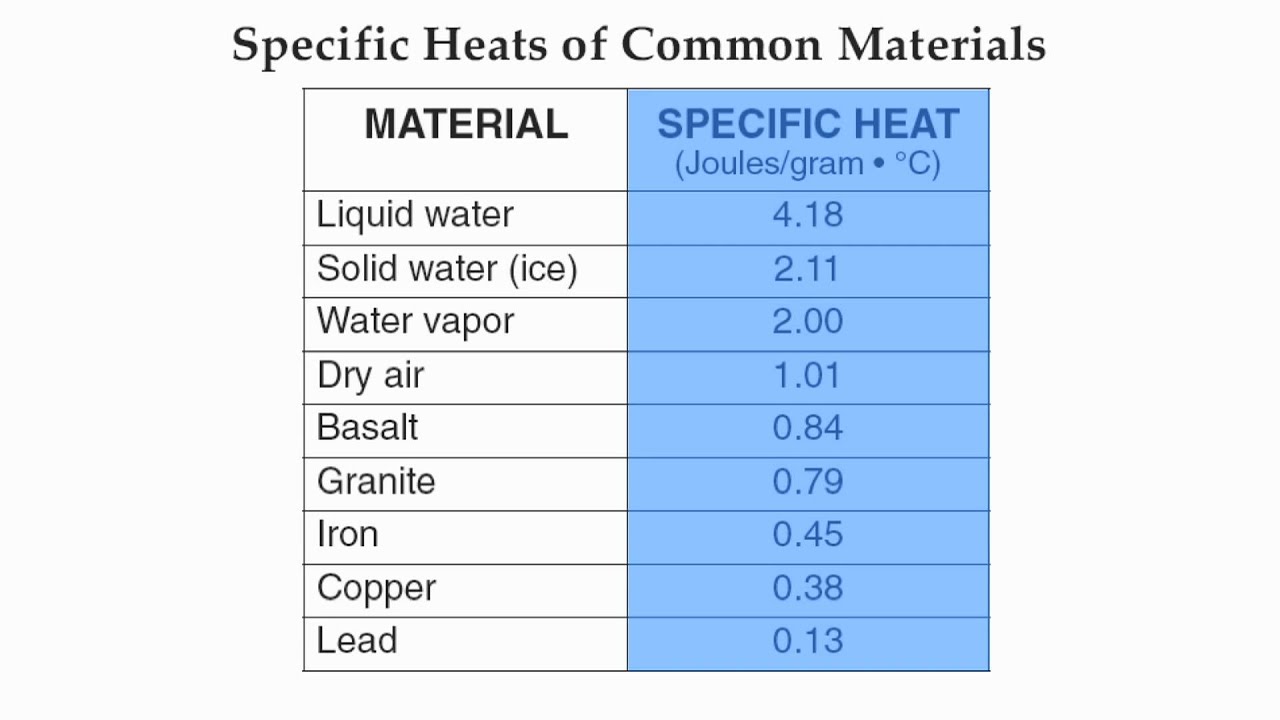


0 Response to "43 What Is The Specific Heat Of Liquid Water"
Post a Comment