40 Rate And Order Of H2o2 Decomposition Lab Answers
studyres.com › doc › 13271281question bank - Directorate of Education - Studyres Thank you for your participation! * Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project Lab 11 - Chemical Kinetics - WebAssign The purpose of the experiment is to measure the rate of Reaction 6. A product of this redox reaction between iodide ions and persulfate ions (S 2 O 8 2-) is elemental iodine.Iodine, in the presence of thiosulfate ions (S 2 O 3 2-) will reform iodide ions (Reaction 7).Iodine can also, in the presence of starch, form a blue-black product (Reaction 8).
science.gov › topicpages › ccabbage juice indicator: Topics by Science.gov Fruits, vegetables, 100% juices, and cognitive function.. PubMed. Lamport, Daniel J; Saunders, Caroline; Butler, Laurie T; Spencer, Jeremy Pe. 2014-12-01. Although reviews of the association between polyphenol intake and cognition exist, research examining the cognitive effects of fruit, vegetable, and juice consumption across epidemiological and intervention studies has not been previously ...

Rate and order of h2o2 decomposition lab answers
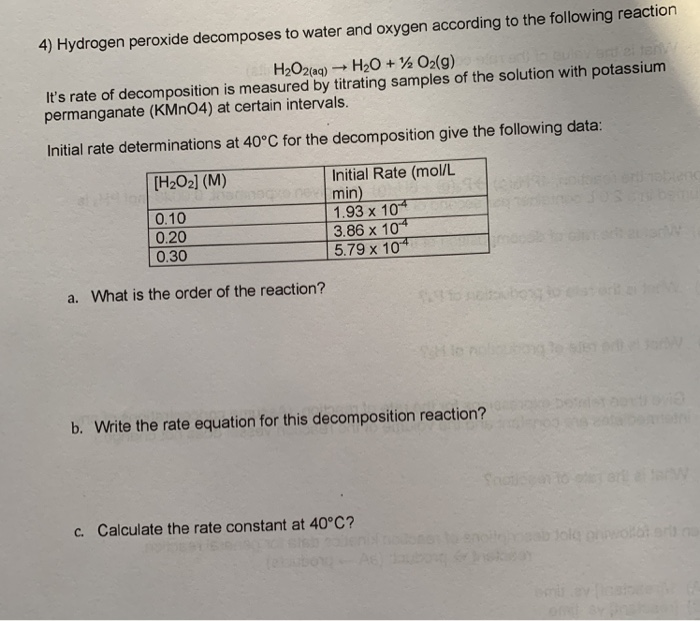
18. Kinetics of Hydrogen Peroxide Decomposition ... The decomposition is characterized by the stoichiometric reaction 1. 2I − + 2H + + H2O2 → I2 + 2H2O If the solution is relatively acidic (with a pH is less than about 3) the rate of reaction 1 is independent of the pH. Assume that the rate of the reaction under acidic conditions is given by Equation 2. Rate = k[I −]a[H2O2]b › document › 363747258General Chemistry 1 PDF | PDF | Gases | Molecules - Scribd (LAB)Determine the effect of various STEM_GC11CK-IIIi-j-139 factors on the rate of a reaction Fourth Quarter General Chemistry 2 Chemical Thermodynamics spontaneous change, prepare a poster on a 1. predict the spontaneity of a process based 1. HELP needed: RE Decomposition of Hydrogen Peroxide - The ... Remember rate will be affected by temperature and pH, so unless you are investigating these variables also you will need to keep these constant throughout. Calorimetry - decomposition of hydrogen peroxide will result in a temperature change - you can use the calorimeter to measure the rate of change in the same way you are measuring the rate of oxygen evolution.
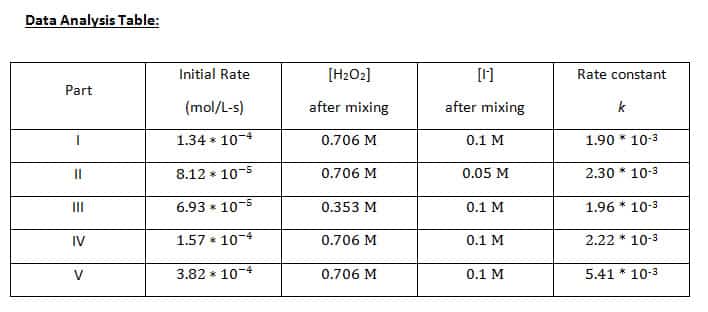
Rate and order of h2o2 decomposition lab answers. Using Graphs to Determine Rate Laws, Rate Constants, and ... This means that the decomposition of N 2 O 5 is first order in [N 2 O 5]. B The rate law for the reaction is therefore. rate = k[N 2 O 5] Calculating the rate constant is straightforward because we know that the slope of the plot of ln[A] versus t for a first-order reaction is −k. Rate Of Reaction Lab Report - 946 Words | Internet Public ... Hypothesis 1: Decrease in concentration of hydrogen peroxide (H2O2) decreases the rate of reaction (that is, increases the time for reaction to come to completion). In the reaction between potassium iodide (KI), hydrogen peroxide, Sodium thiosulfate (Na2S2O4) under acidic condition. lab 4 report - Rate of decomposition for Hydrogen Peroxide ... Data Analysis Table: [H 2 O 2] M [ I-] M Rate ml/s Experiment 1 0.15 0.033 4.29 x 10-2 Experiment 2 0.294 0.033 5.53 x 10-2 Experiment 3 0.15 0.067 4.86 x 10-2 Rate= k [H 2 O 2] a [I-] b The order of the concentration of H 2 O 2: LAB 4 Reaction Rates - LAB 4: Reaction Rates CHEM 1161 I ... RESULTS Overall rate law: K [ H 2 O 2 ]^1 * [IKI]^1 IV. CONCLUSION Order of H2O2 and IKI were determined that both were of first order. Hence total order of reaction is 1 + 1 = 2. Therefore, the overall it is an II order reaction. V. QUESTIONS 1.
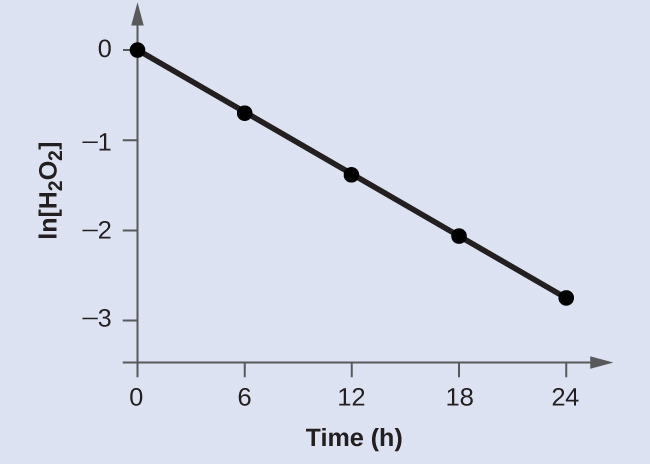
Decomposition Of Hydrogen Peroxide Lab Report - Cram.com They increase the rate of a reaction without undergoing a net change. The change in pressure will determined by the LabQuest. The objectives of this experiment are to monitor the rate of the chemical reaction, determine kinetic order of a reaction from the dependence of the rate on reactant concentrations. Kinetic order can be found by using this calculation Rate=k〖[H_2 O_2]〗^m 〖[KI]〗^n PDF Chemical Kinetics Reaction Rates The Overall Order of a reaction is the sum of the individual orders: Rate (Ms−1) = k[A][B]1/2[C]2 Overall order: 1 + ½ + 2 = 3.5 = 7/2 or seven−halves order note: when the order of a reaction is 1 (first order) no exponent is written. Units for the rate constant: The units of a rate constant will change depending upon the overall order. 12.1 Chemical Reaction Rates - Chemistry The rate of decomposition of H 2 O 2 in an aqueous solution decreases as the concentration of H 2 O 2 decreases. To obtain the tabulated results for this decomposition, the concentration of hydrogen peroxide was measured every 6 hours over the course of a day at a constant temperature of 40 °C. › 37304387 › Water_and_WastewaterWater and Wastewater Calculations Manual 2nd ... - Academia.edu Academia.edu is a platform for academics to share research papers.
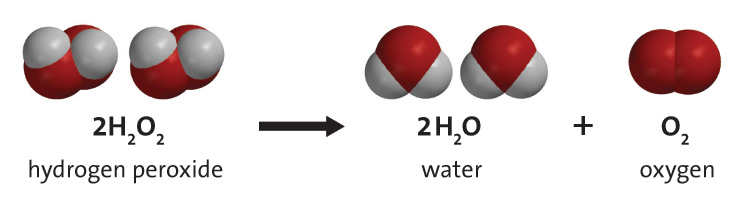
Experiment 17: The Rate and Order of a Chemical Reaction ... The absorbance of a particular wavelength of light by a solution. 2) In this experiment you will conduct the reaction between solutions of potassium iodide and iron (III) chloride. The reaction equation is shown below, in ionic form. 2 I - (aq) + 2 Fe 3+ (aq) → I 2 (aq) + 2 Fe 2+ (aq) Rate of Reaction Lab Report by Harsha Pillai - Prezi 1. Measure 50cm^3 of Hydrogen Peroxide by using the graduated cylinder. 2. Using the scale measure the mass of the 250mL beaker and record numerical value. 3. Pour 25cm^3 of Hydrogen Peroxide into the 250mL beaker and again using the scale weigh the mass. Keep the beaker on the scale throughout the entire experiment. 4. PDF Lab 5 Iodine Clock - University of Massachusetts Boston Row 6: Ratei is the initial rate of the reaction: Δ[H2O2]/Δt Row 7, 8 and 9: Use the initial rate and [H2O2]i to calculate a k′ value assuming the order, x = 0, 1, or 2 k′ = Ratei / [H2O2]i Row 10: Determine the order in H2O2 by examining the calculated k′s calculated in row 7, 8 and 9 for the first three trials. Decomposition of Hydrogen Peroxide Lab Answers ... The reaction that occurred during this lab was the decomposition of hydrogen peroxide catalyzed with the presence of potassium iodide. The decomposition of hydrogen peroxide by itself is 2H 2 O 2 (aq) -> 2H 2 O (l) + O 2 (g). However, this was not the exact reaction that took place.
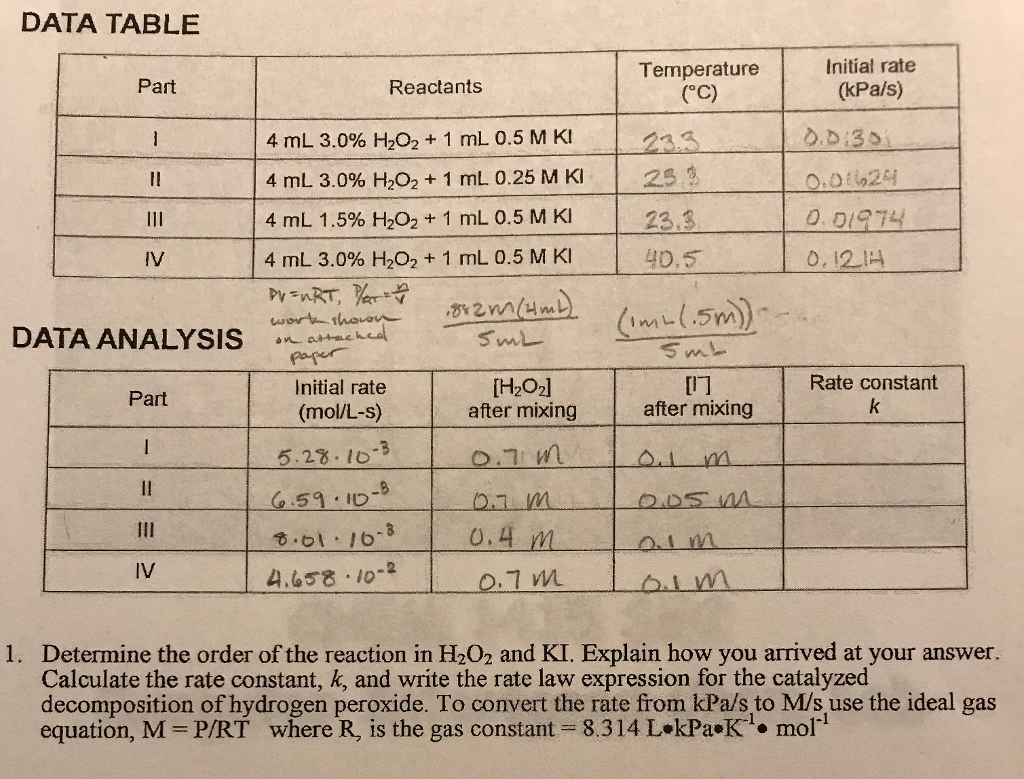
Solved 30 Pre-lab Questions Rates of Chemical Reactions II ... Question: 30 Pre-lab Questions Rates of Chemical Reactions II: Rate and Order of H2O2 Decomposition Before beginning this experiment in the laboratory, you should be able to answer the following questions. 1. What six factors may influence the rate of a reaction? 2. A B . Assume that the rate law for a reaction is rate= (a) What is the overall order of the reaction?
Chemical Kinetics Class 12 NCSERT Solutions - NCERT help Calculate the initial rate of the reaction when [A] = 0.1 mol L-1, [B] = 0.2 mol L-1. Calculate the rate of reaction after [A] is reduced to 0.06 mol L-1. Question 4.3 The decomposition of NH3 on platinum surface is zero order reaction. What are the rates of production of N2 and H2 if k = 2.5 × 10-4 mol-1 L s-1?
PDF Experiment 5 Kinetics: The Oxidation of Iodide by Hydrogen ... The reaction is said to be first order in A and second order in B. Overall, the order of the reaction is 3. The value of the rate constant k can be determined by using the known values of n and m: 2 Rate k= [A][B] We can use the given initial concentrations and initial rate for each experiment and determine the value of k for each experiment.
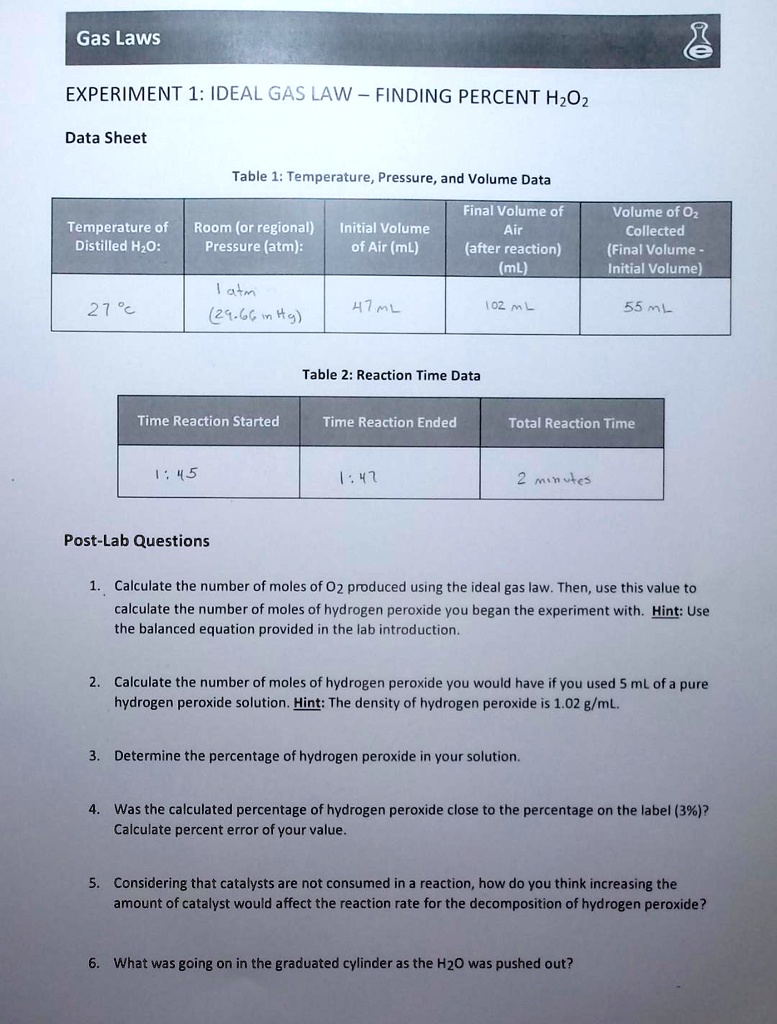
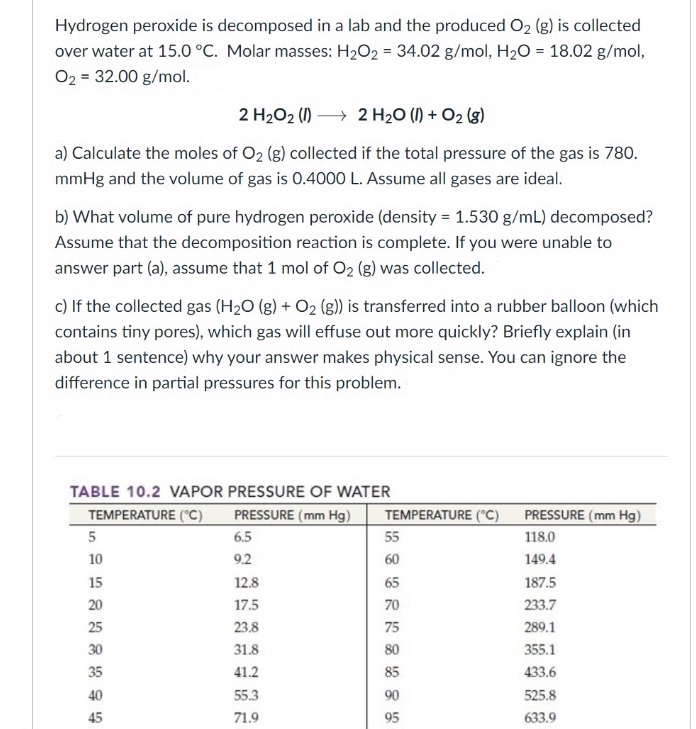
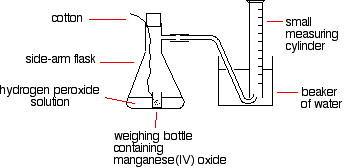
DOC 28. Determination of the Rate of the Decomposition of ... Every 2 H2O2 molecules yields one O2 molecule; therefore, the rate at which H2O2 disappears is half the rate at which O2 is formed: Because the concentration of oxygen is proportional to its pressure, we can calculate the rate at which H2O2 decomposes by monitoring the rate of increase of the pressure due to the formation of oxygen.
› category › tvTV Archives - Hollywood.com Click to get the latest TV content. Sign up for your weekly dose of feel-good entertainment and movie content!
PPT Chemical Ideas 10.3 rate = k [A]m [B]n m and n are powers to which the concentration must be raised. usually have values of 0, 1 or 2. m & n are called the order of the reaction decomposition of hydrogen peroxide rate = k [H2O2(aq)] [catalase] The reaction is first order with respect to H2O2 The reaction is also first order with respect to catalase.
Lab report the kinetics of the reaction by Yufei Chang - Issuu [A]n [A] is the concentration of A at time t t is the time k is the rate constant n is the order of reaction If the reaction is pseudo first order with respect to H2O2: or
PDF The Decomposition of Hydrogen Peroxide - Chem21Labs the relationship between the rate and the concentration of the reactants is given by the rate law Rate k [A]m [B]n In this rate law equation, k is a constant, called the rate constant of the reaction and should be the same for trials of the reaction at a given temperature. The superscripts, m and n, are the order of the reaction with
Solved Rates of chemical reactions II: rate and order of ... Vapor pressure f bath temerature T = 17°C --> from databases P = 12.7931 torr Compared to the rate found in solution 1... the rate should be --> double, s …. View the full answer. Transcribed image text: Chemical Reactions II: Rate and Order of H202 Decomposition A. Order of Reaction BATH TEMPERATURE oC oc °C Soluti Buret reading (mL) Buret Buret ...
mary-beauty.de › hclrsLaki-laki Ini Berupaya Setubuhi Tetangga ... - mary-beauty.de 2 days ago · Preparation of Enzyme Extracts Each table will process one russet as well as one other type of 5. lab enzyme answers. This is an biology lab report example about enzyme concentration and the activity of catalase. The bubbling reaction you see is the metabolic process of decomposition, described earlier. temp & potato catalase rxn rate results ...
Lab quiz 4: Stoichiometric Calculations and Reaction Rates ... For example, the rate law for the decomposition of H2O2 is expressed by the following equation:-Because the reaction rate is directly proportional to the concentration of H2O2 raised to the first power (k [H2O2]1), the decomposition of H2O2 is first order for H2O2.
Calculations for Rate and Order of H2O2 Lab - YouTube About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
5.2: Methods of Determining Reaction Order - Chemistry ... The differential rate for a first-order reaction is as follows: (14.19) rate = − Δ [ A] Δ t = k [ A] If the concentration of A is doubled, the reaction rate doubles; if the concentration of A is increased by a factor of 10, the reaction rate increases by a factor of 10, and so forth.

Decomposition of H2O2 follows a first order reaction. In fifty minutes the concentration of H2O2 decreases from 0.5 to 0.125 M in one such decomposition. When the concentration of H2O2 reaches 0.05 M, ...
Decomposition of H2O2 is a first order reaction. A ... Decomposition of H2O2 is a first order reaction. A solution of H2O2... You are viewing a single answer. Ex Senior Faculty of Resonance Kota and Bansal Classes Kota. Decomposition of H2O2 is a first order reaction. A solution of H2O2 labelled as "16.8V" was left open. Due to this, s...
The rate law for the reaction H2O2 + 2H+ + 2l- → I2 ... Given that the rate law for the production of water 2 H2(g) + O2(g) → 2H2O(l) is rate = k[H2]2[O2]-1, then the overall order of the reaction is a)... Q. If the rate of a reaction increases by a factor of 9 when the concentration of reactant increases by a factor of 3, the order of the reaction (with...
› 17546075 › Corrosion_EngineeringCorrosion Engineering : Principles and Practice - Academia.edu Academia.edu is a platform for academics to share research papers.
Question:how does pH affect decomposion of H2O2 ... I really need help on this lab. it's a gr.12 chemistry lab, i chose the topic of how does the pH affect the rate of catalytic decomposition of H2O2, i'm using KI as the catalyst, and adding HCl or NaOH to change the pH. all we have to do is just the planning part, i need help on explaining why and how the pH of the mixture affect the rate of decomposition of H2O2.
The rate constant for the first-order decomposition of ... Order of the reaction = First order. t 1/2 = 200 minutes = 200 × 60 = 12,000 seconds The relation between t 1/2. and k is given by t 1/2 = 0.693/k. k = .693/12000 = 5.7 × 10−5. The rate constant for the first-order decomposition of H 2 O 2 is given by. 2897 Views.
HELP needed: RE Decomposition of Hydrogen Peroxide - The ... Remember rate will be affected by temperature and pH, so unless you are investigating these variables also you will need to keep these constant throughout. Calorimetry - decomposition of hydrogen peroxide will result in a temperature change - you can use the calorimeter to measure the rate of change in the same way you are measuring the rate of oxygen evolution.
› document › 363747258General Chemistry 1 PDF | PDF | Gases | Molecules - Scribd (LAB)Determine the effect of various STEM_GC11CK-IIIi-j-139 factors on the rate of a reaction Fourth Quarter General Chemistry 2 Chemical Thermodynamics spontaneous change, prepare a poster on a 1. predict the spontaneity of a process based 1.
18. Kinetics of Hydrogen Peroxide Decomposition ... The decomposition is characterized by the stoichiometric reaction 1. 2I − + 2H + + H2O2 → I2 + 2H2O If the solution is relatively acidic (with a pH is less than about 3) the rate of reaction 1 is independent of the pH. Assume that the rate of the reaction under acidic conditions is given by Equation 2. Rate = k[I −]a[H2O2]b

















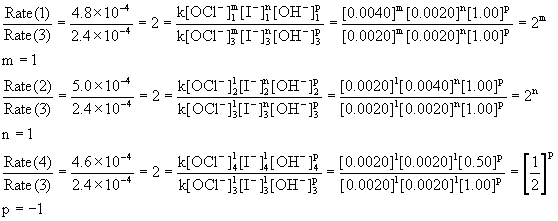


0 Response to "40 Rate And Order Of H2o2 Decomposition Lab Answers"
Post a Comment